Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis
- PMID: 24982181
- PMCID: PMC4104921
- DOI: 10.1073/pnas.1407777111
Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis
Abstract
Ablation of a single miRNA gene rarely leads to a discernable developmental phenotype in mice, in some cases because of compensatory effects by other functionally related miRNAs. Here, we report that simultaneous inactivation of two functionally related miRNA clusters (miR-34b/c and miR-449) encoding five miRNAs (miR-34b, miR-34c, miR-449a, miR-449b, and miR-449c) led to sexually dimorphic, partial perinatal lethality, growth retardation, and infertility. These developmental defects correlated with the dysregulation of ∼ 240 target genes, which are mainly involved in three major cellular functions, including cell-fate control, brain development and microtubule dynamics. Our data demonstrate an essential role of a miRNA family in brain development, motile ciliogenesis, and spermatogenesis.
Keywords: airway obstruction; egg transport; forebrain; oviduct.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
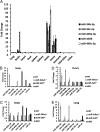
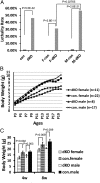

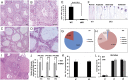
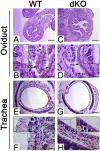
References
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. - PubMed
-
- Janga SC, Vallabhaneni S. MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv Exp Med Biol. 2011;722:59–74. - PubMed
-
- Peters J, Robson JE. Imprinted noncoding RNAs. Mamm Genome. 2008;19(7-8):493–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS077169/NS/NINDS NIH HHS/United States
- P20-GM103650/GM/NIGMS NIH HHS/United States
- P20 GM103440/GM/NIGMS NIH HHS/United States
- HD074573/HD/NICHD NIH HHS/United States
- P20 GM103554/GM/NIGMS NIH HHS/United States
- HD071736/HD/NICHD NIH HHS/United States
- R01 HD060858/HD/NICHD NIH HHS/United States
- P20 RR018751/RR/NCRR NIH HHS/United States
- P20-RR18751/RR/NCRR NIH HHS/United States
- P20-GM103554/GM/NIGMS NIH HHS/United States
- P20 RR016464/RR/NCRR NIH HHS/United States
- R21 HD071736/HD/NICHD NIH HHS/United States
- P20-GM103440/GM/NIGMS NIH HHS/United States
- P20 GM103650/GM/NIGMS NIH HHS/United States
- HD060858/HD/NICHD NIH HHS/United States
- R03 HD074573/HD/NICHD NIH HHS/United States
- R21 NS077169/NS/NINDS NIH HHS/United States
- P20-RR016464/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

