Activity-dependent dendritic spine neck changes are correlated with synaptic strength
- PMID: 24982196
- PMCID: PMC4104910
- DOI: 10.1073/pnas.1321869111
Activity-dependent dendritic spine neck changes are correlated with synaptic strength
Abstract
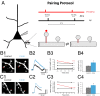
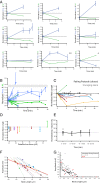
Most excitatory inputs in the mammalian brain are made on dendritic spines, rather than on dendritic shafts. Spines compartmentalize calcium, and this biochemical isolation can underlie input-specific synaptic plasticity, providing a raison d'etre for spines. However, recent results indicate that the spine can experience a membrane potential different from that in the parent dendrite, as though the spine neck electrically isolated the spine. Here we use two-photon calcium imaging of mouse neocortical pyramidal neurons to analyze the correlation between the morphologies of spines activated under minimal synaptic stimulation and the excitatory postsynaptic potentials they generate. We find that excitatory postsynaptic potential amplitudes are inversely correlated with spine neck lengths. Furthermore, a spike timing-dependent plasticity protocol, in which two-photon glutamate uncaging over a spine is paired with postsynaptic spikes, produces rapid shrinkage of the spine neck and concomitant increases in the amplitude of the evoked spine potentials. Using numerical simulations, we explore the parameter regimes for the spine neck resistance and synaptic conductance changes necessary to explain our observations. Our data, directly correlating synaptic and morphological plasticity, imply that long-necked spines have small or negligible somatic voltage contributions, but that, upon synaptic stimulation paired with postsynaptic activity, they can shorten their necks and increase synaptic efficacy, thus changing the input/output gain of pyramidal neurons.
Keywords: STDP; basal dendrites; neocortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ramón y Cajal S. 1899. La Textura del Sistema Nerviosa del Hombre y los Vertebrados (Moya, Madrid)
-
- Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183(4675):1592–1593. - PubMed
-
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9(2):268–287. - PubMed
-
- Arellano JI, Espinosa A, Fairén A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145(2):464–469. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

