Biochemical characterization of a dihydroneopterin aldolase used for methanopterin biosynthesis in methanogens
- PMID: 24982305
- PMCID: PMC4135653
- DOI: 10.1128/JB.01812-14
Biochemical characterization of a dihydroneopterin aldolase used for methanopterin biosynthesis in methanogens
Abstract
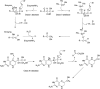
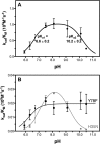
The gene encoding 7,8-dihydroneopterin aldolase (DHNA) was recently identified in archaea through comparative genomics as being involved in methanopterin biosynthesis (V. Crécy-Lagard, G. Phillips, L. L. Grochowski, B. El Yacoubi, F. Jenney, M. W. Adams, A. G. Murzin, and R. H. White, ACS Chem. Biol. 7:1807-1816, 2012, doi:10.1021/cb300342u). Archaeal DHNA shows a unique secondary and quaternary structure compared with bacterial and plant DHNAs. Here, we report a detailed biochemical examination of DHNA from the methanogen Methanocaldococcus jannaschii. Kinetic studies show that M. jannaschii DHNA possesses a catalytic capability with a kcat/Km above 10(5) M(-1) s(-1) at 70°C, and at room temperature it exhibits a turnover number (0.07 s(-1)) comparable to bacterial DHNAs. We also found that this enzyme follows an acid-base catalytic mechanism similar to the bacterial DHNAs, except when using alternative catalytic residues. We propose that in the absence of lysine, which is considered to be the general base in bacterial DHNAs, an invariant water molecule likely functions as the catalytic base, and the strictly conserved His35 and Gln61 residues serve as the hydrogen bond partners to adjust the basicity of the water molecule. Indeed, substitution of either His35 or Gln61 causes a 20-fold decrease in kcat. An invariant Tyr78 is also shown to be important for catalysis, likely functioning as a general acid. Glu25 plays an important role in substrate binding, since replacing Glu25 by Gln caused a ≥25-fold increase in Km. These results provide important insights into the catalytic mechanism of archaeal DHNAs.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
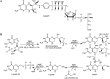

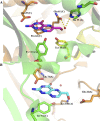
References
-
- Neue H. 1993. Methane emissions from rice fields. Bioscience 43:466–476. 10.2307/1311906 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

