Accurate phosphoregulation of kinetochore-microtubule affinity requires unconstrained molecular interactions
- PMID: 24982430
- PMCID: PMC4085703
- DOI: 10.1083/jcb.201312107
Accurate phosphoregulation of kinetochore-microtubule affinity requires unconstrained molecular interactions
Abstract
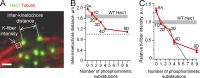
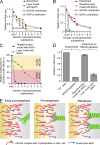
Accurate chromosome segregation relies on dynamic interactions between microtubules (MTs) and the NDC80 complex, a major kinetochore MT-binding component. Phosphorylation at multiple residues of its Hec1 subunit may tune kinetochore-MT binding affinity for diverse mitotic functions, but molecular details of such phosphoregulation remain elusive. Using quantitative analyses of mitotic progression in mammalian cells, we show that Hec1 phosphorylation provides graded control of kinetochore-MT affinity. In contrast, modeling the kinetochore interface with repetitive MT binding sites predicts a switchlike response. To reconcile these findings, we hypothesize that interactions between NDC80 complexes and MTs are not constrained, i.e., the NDC80 complexes can alternate their binding between adjacent kinetochore MTs. Experiments using cells with phosphomimetic Hec1 mutants corroborate predictions of such a model but not of the repetitive sites model. We propose that accurate regulation of kinetochore-MT affinity is driven by incremental phosphorylation of an NDC80 molecular "lawn," in which the NDC80-MT bonds reorganize dynamically in response to the number and stability of MT attachments.
© 2014 Zaytsev et al.
Figures




References
-
- Alberts, B., Johnson A., Lewis J., Raff M., Roberts K., and Walter P.. 2008. Molecular Biology of the Cell. Fifth edition Garland Science, New York: 1392 pp
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

