Dopamine: a parallel pathway for the modulation of spinal locomotor networks
- PMID: 24982614
- PMCID: PMC4059167
- DOI: 10.3389/fncir.2014.00055
Dopamine: a parallel pathway for the modulation of spinal locomotor networks
Abstract
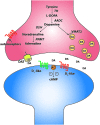
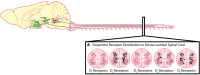
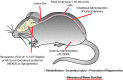
The spinal cord contains networks of neurons that can produce locomotor patterns. To readily respond to environmental conditions, these networks must be flexible yet at the same time robust. Neuromodulators play a key role in contributing to network flexibility in a variety of invertebrate and vertebrate networks. For example, neuromodulators contribute to altering intrinsic properties and synaptic weights that, in extreme cases, can lead to neurons switching between networks. Here we focus on the role of dopamine in the control of stepping networks in the spinal cord. We first review the role of dopamine in modulating rhythmic activity in the stomatogastric ganglion (STG) and the leech, since work from these preparations provides a foundation to understand its role in vertebrate systems. We then move to a discussion of dopamine's role in modulation of swimming in aquatic species such as the larval xenopus, lamprey and zebrafish. The control of terrestrial walking in vertebrates by dopamine is less studied and we review current evidence in mammals with a focus on rodent species. We discuss data suggesting that the source of dopamine within the spinal cord is mainly from the A11 area of the diencephalon, and then turn to a discussion of dopamine's role in modulating walking patterns from both in vivo and in vitro preparations. Similar to the descending serotonergic system, the dopaminergic system may serve as a potential target to promote recovery of locomotor function following spinal cord injury (SCI); evidence suggests that dopaminergic agonists can promote recovery of function following SCI. We discuss pharmacogenetic and optogenetic approaches that could be deployed in SCI and their potential tractability. Throughout the review we draw parallels with both noradrenergic and serotonergic modulatory effects on spinal cord networks. In all likelihood, a complementary monoaminergic enhancement strategy should be deployed following SCI.
Keywords: central pattern generator; dopamine; locomotion; monoamines; spinal cord.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

