The physiological functions of iron regulatory proteins in iron homeostasis - an update
- PMID: 24982634
- PMCID: PMC4056636
- DOI: 10.3389/fphar.2014.00124
The physiological functions of iron regulatory proteins in iron homeostasis - an update
Abstract
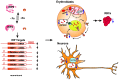
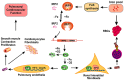
Iron regulatory proteins (IRPs) regulate the expression of genes involved in iron metabolism by binding to RNA stem-loop structures known as iron responsive elements (IREs) in target mRNAs. IRP binding inhibits the translation of mRNAs that contain an IRE in the 5'untranslated region of the transcripts, and increases the stability of mRNAs that contain IREs in the 3'untranslated region of transcripts. By these mechanisms, IRPs increase cellular iron absorption and decrease storage and export of iron to maintain an optimal intracellular iron balance. There are two members of the mammalian IRP protein family, IRP1 and IRP2, and they have redundant functions as evidenced by the embryonic lethality of the mice that completely lack IRP expression (Irp1 (-/-)/Irp2(-/-) mice), which contrasts with the fact that Irp1 (-/-) and Irp2 (-/-) mice are viable. In addition, Irp2 (-/-) mice also display neurodegenerative symptoms and microcytic hypochromic anemia, suggesting that IRP2 function predominates in the nervous system and erythropoietic homeostasis. Though the physiological significance of IRP1 had been unclear since Irp1 (-/-) animals were first assessed in the early 1990s, recent studies indicate that IRP1 plays an essential function in orchestrating the balance between erythropoiesis and bodily iron homeostasis. Additionally, Irp1 (-/-) mice develop pulmonary hypertension, and they experience sudden death when maintained on an iron-deficient diet, indicating that IRP1 has a critical role in the pulmonary and cardiovascular systems. This review summarizes recent progress that has been made in understanding the physiological roles of IRP1 and IRP2, and further discusses the implications for clinical research on patients with idiopathic polycythemia, pulmonary hypertension, and neurodegeneration.
Keywords: erythropoiesis; iron metabolism; iron regulatory protein; iron responsive element; polycythemia; pulmonary hypertension.
Figures
References
-
- Brusselmans K., Compernolle V., Tjwa M., Wiesener M. S., Maxwell P. H., Collen D., et al. (2003). Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J. Clin. Invest. 111 1519–1527 10.1172/JCI15496 - DOI - PMC - PubMed
-
- Bushuev V. I., Miasnikova G. Y., Sergueeva A. I., Polyakova L. A., Okhotin D., Gaskin P. R., et al. (2006). Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica 91 744–749 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

