The FMRFamide-Like Peptide Family in Nematodes
- PMID: 24982652
- PMCID: PMC4058706
- DOI: 10.3389/fendo.2014.00090
The FMRFamide-Like Peptide Family in Nematodes
Erratum in
-
Corrigendum: the FMRFamide-like peptide family in nematodes.Front Neurosci. 2015 Apr 9;9:120. doi: 10.3389/fnins.2015.00120. eCollection 2015. Front Neurosci. 2015. PMID: 25914615 Free PMC article.
Abstract
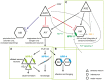
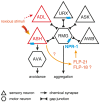
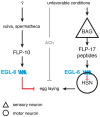
In the three decades since the FMRFamide peptide was isolated from the mollusk Macrocallista nimbosa, structurally similar peptides sharing a C-terminal RFamide motif have been identified across the animal kingdom. FMRFamide-like peptides (FLPs) represent the largest known family of neuropeptides in invertebrates. In the phylum Nematoda, at least 32 flp-genes are classified, making the FLP system of nematodes unusually complex. The diversity of the nematode FLP complement is most extensively mapped in Caenorhabditis elegans, where over 70 FLPs have been predicted. FLPs have shown to be expressed in the majority of the 302 C. elegans neurons including interneurons, sensory neurons, and motor neurons. The vast expression of FLPs is reflected in the broad functional repertoire of nematode FLP signaling, including neuroendocrine and neuromodulatory effects on locomotory activity, reproduction, feeding, and behavior. In contrast to the many identified nematode FLPs, only few peptides have been assigned a receptor and there is the need to clarify the pathway components and working mechanisms of the FLP signaling network. Here, we review the diversity, distribution, and functions of FLPs in nematodes.
Keywords: C. elegans; FMRFamide-like peptides; G protein-coupled receptor; feeding behavior; nematodes; neuropeptide; reproduction.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

