Protective immunity against hepatitis C: many shades of gray
- PMID: 24982656
- PMCID: PMC4058636
- DOI: 10.3389/fimmu.2014.00274
Protective immunity against hepatitis C: many shades of gray
Abstract
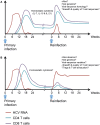
The majority of individuals who become acutely infected with hepatitis C virus (HCV) develop chronic infection and suffer from progressive liver damage while approximately 25% are able to eliminate the virus spontaneously. Despite the recent introduction of new direct-acting antivirals, there is still no vaccine for HCV. As a result, new infections and reinfections will remain a problem in developing countries and among high risk populations like injection drug users who have limited access to treatment and who continue to be exposed to the virus. The outcome of acute HCV is determined by the interplay between the host genetics, the virus, and the virus-specific immune response. Studies in humans and chimpanzees have demonstrated the essential role of HCV-specific CD4 and CD8 T cell responses in protection against viral persistence. Recent data suggest that antibody responses play a more important role than what was previously thought. Individuals who spontaneously resolve acute HCV infection develop long-lived memory T cells and are less likely to become persistently infected upon reexposure. New studies examining high risk cohorts are identifying correlates of protection during real life exposures and reinfections. In this review, we discuss correlates of protective immunity during acute HCV and upon reexposure. We draw parallels between HCV and the current knowledge about protective memory in other models of chronic viral infections. Finally, we discuss some of the yet unresolved questions about key correlates of protection and their relevance for vaccine development against HCV.
Keywords: HCV reinfection; NK cells; T cells; acute infection; adaptive immunity; antibodies; hepatitis C; innate immunity.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

