Mechanisms involving Ang II and MAPK/ERK1/2 signaling pathways underlie cardiac and renal alterations during chronic undernutrition
- PMID: 24983243
- PMCID: PMC4077653
- DOI: 10.1371/journal.pone.0100410
Mechanisms involving Ang II and MAPK/ERK1/2 signaling pathways underlie cardiac and renal alterations during chronic undernutrition
Abstract
Background: Several studies have correlated protein restriction associated with other nutritional deficiencies with the development of cardiovascular and renal diseases. The driving hypothesis for this study was that Ang II signaling pathways in the heart and kidney are affected by chronic protein, mineral and vitamin restriction.
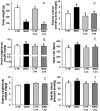
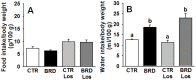
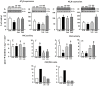
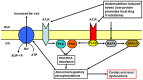
Methodology/principal findings: Wistar rats aged 90 days were fed from weaning with either a control or a deficient diet that mimics those used in impoverished regions worldwide. Such restriction simultaneously increased ouabain-insensitive Na+-ATPase and decreased (Na++K+)ATPase activity in the same proportion in cardiomyocytes and proximal tubule cells. Type 1 angiotensin II receptor (AT1R) was downregulated by that restriction in both organs, whereas AT2R decreased only in the kidney. The PKC/PKA ratio increased in both tissues and returned to normal values in rats receiving Losartan daily from weaning. Inhibition of the MAPK pathway restored Na+-ATPase activity in both organs. The undernourished rats presented expanded plasma volume, increased heart rate, cardiac hypertrophy, and elevated systolic pressure, which also returned to control levels with Losartan. Such restriction led to electrical cardiac remodeling represented by prolonged ventricular repolarization parameters, induced triggered activity, early after-depolarization and delayed after-depolarization, which were also prevented by Losartan.
Conclusion/significance: The mechanisms responsible for these alterations are underpinned by an imbalance in the PKC- and PKA-mediated pathways, with participation of angiotensin receptors and by activation of the MAPK/ERK1/2 pathway. These cellular and molecular alterations culminate in cardiac electric remodeling and in the onset of hypertension in adulthood.
Conflict of interest statement
Figures








References
-
- Monteiro CA, Benicio MH, Konno SC, Silva AC, Lima AL, et al. (2009) Causes for the decline in child under-nutrition in Brazil, 1996–2007. Rev Saude Publica 43: 35–43. - PubMed
-
- Fowden AL, Giussani DA, Forhead AJ (2006) Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21: 29–37. - PubMed
-
- Fernandez-Twinn DS, Ekizoglou S, Wayman A, Petry CJ, Ozanne SE (2006) Maternal low-protein diet programs cardiac beta-adrenergic response and signaling in 3-mo-old male offspring. Am J Physiol Regul Integr Comp Physiol 291: R429–436. - PubMed
-
- Langley-Evans SC, Welham SJ, Jackson AA (1999) Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

