Factor XIII activity mediates red blood cell retention in venous thrombi
- PMID: 24983320
- PMCID: PMC4109540
- DOI: 10.1172/JCI75386
Factor XIII activity mediates red blood cell retention in venous thrombi
Abstract
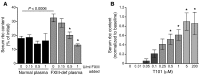
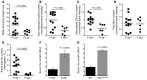
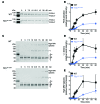
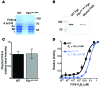
Venous thrombi, fibrin- and rbc-rich clots triggered by inflammation and blood stasis, underlie devastating, and sometimes fatal, occlusive events. During intravascular fibrin deposition, rbc are thought to become passively trapped in thrombi and therefore have not been considered a modifiable thrombus component. In the present study, we determined that activity of the transglutaminase factor XIII (FXIII) is critical for rbc retention within clots and directly affects thrombus size. Compared with WT mice, mice carrying a homozygous mutation in the fibrinogen γ chain (Fibγ390-396A) had a striking 50% reduction in thrombus weight due to reduced rbc content. Fibrinogen from mice harboring the Fibγ390-396A mutation exhibited reduced binding to FXIII, and plasma from these mice exhibited delayed FXIII activation and fibrin crosslinking, indicating these residues mediate FXIII binding and activation. FXIII-deficient mice phenocopied mice carrying Fibγ390-396A and produced smaller thrombi with fewer rbc than WT mice. Importantly, FXIII-deficient human clots also exhibited reduced rbc retention. The addition of FXIII to FXIII-deficient clots increased rbc retention, while inhibition of FXIII activity in normal blood reduced rbc retention and produced smaller clots. These findings establish the FXIII-fibrinogen axis as a central determinant in venous thrombogenesis and identify FXIII as a potential therapeutic target for limiting venous thrombosis.
Figures



References
-
- Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

