A novel restricted diffusion model of evoked dopamine
- PMID: 24983330
- PMCID: PMC4176316
- DOI: 10.1021/cn5000666
A novel restricted diffusion model of evoked dopamine
Abstract
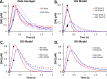
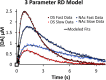
In vivo fast-scan cyclic voltammetry provides high-fidelity recordings of electrically evoked dopamine release in the rat striatum. The evoked responses are suitable targets for numerical modeling because the frequency and duration of the stimulus are exactly known. Responses recorded in the dorsal and ventral striatum of the rat do not bear out the predictions of a numerical model that assumes the presence of a diffusion gap interposed between the recording electrode and nearby dopamine terminals. Recent findings, however, suggest that dopamine may be subject to restricted diffusion processes in brain extracellular space. A numerical model cast to account for restricted diffusion produces excellent agreement between simulated and observed responses recorded under a broad range of anatomical, stimulus, and pharmacological conditions. The numerical model requires four, and in some cases only three, adjustable parameters and produces meaningful kinetic parameter values.
Keywords: Model; diffusion; domain; dopamine; restricted diffusion; voltammetry.
Figures




References
-
- Schultz W. (2007) Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288. - PubMed
-
- Schultz W. (1982) Depletion of dopamine in the striatum as an experimental model of Parkinsonism: direct effects and adaptive mechanisms. Prog. Neurobiol. (Oxford, U.K.) 18, 121–166. - PubMed
-
- Sagvolden T.; Johansen E. B.; Aase H.; Russell V. A. (2005) A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 28, 397–419. - PubMed
-
- Carlsson A.; Lindqvist M.; Magnusson T.; Waldeck B. (1958) On the presence of 3-hydroxytyramine in brain. Science 127, 471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

