64Cu-labeled somatostatin analogues conjugated with cross-bridged phosphonate-based chelators via strain-promoted click chemistry for PET imaging: in silico through in vivo studies
- PMID: 24983404
- PMCID: PMC4261236
- DOI: 10.1021/jm500416f
64Cu-labeled somatostatin analogues conjugated with cross-bridged phosphonate-based chelators via strain-promoted click chemistry for PET imaging: in silico through in vivo studies
Abstract
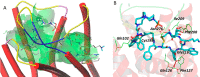
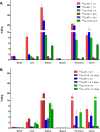
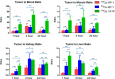
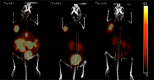
Somatostatin receptor subtype 2 (sstr2) is a G-protein-coupled receptor (GPCR) that is overexpressed in neuroendocrine tumors. The homology model of sstr2 was built and was used to aid the design of new somatostatin analogues modified with phosphonate-containing cross-bridged chelators for evaluation of using them as PET imaging radiopharmaceuticals. The new generation chelators were conjugated to Tyr3-octreotate (Y3-TATE) through bioorthogonal, strain-promoted alkyne azide cycloaddition (SPAAC) to form CB-TE1A1P-DBCO-Y3-TATE (AP) and CB-TE1K1P-PEG4-DBCO-Y3-TATE (KP) in improved yields compared to standard direct conjugation methods of amide bond formation. Consistent with docking studies, the clicked bioconjugates showed high binding affinities to sstr2, with Kd values ranging from 0.6 to 2.3 nM. Selected isomers of the clicked products were used in biodistribution and PET/CT imaging. Introduction of the bulky dibenzocyclooctyne group in AP decreased clearance rates from circulation. However, the additional carboxylate group and PEG linker from the KP conjugate significantly improved labeling conditions and in vivo stability of the copper complex and ameliorated the slower pharmacokinetics of the clicked somatostatin analogues.
Figures

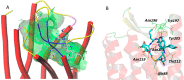


Similar articles
-
Cu(I)-assisted click chemistry strategy for conjugation of non-protected cross-bridged macrocyclic chelators to tumour-targeting peptides.Dalton Trans. 2015 Mar 7;44(9):3945-8. doi: 10.1039/c4dt03897e. Dalton Trans. 2015. PMID: 25645688 Free PMC article.
-
Preparation and biological evaluation of copper-64-labeled tyr3-octreotate using a cross-bridged macrocyclic chelator.Clin Cancer Res. 2004 Dec 15;10(24):8674-82. doi: 10.1158/1078-0432.CCR-04-1084. Clin Cancer Res. 2004. PMID: 15623652
-
Positron emission tomographic imaging of copper 64- and gallium 68-labeled chelator conjugates of the somatostatin agonist tyr3-octreotate.Mol Imaging. 2014;13:DOI 10.2310/7290.2014.00020. doi: 10.2310/7290.2014.00020. Mol Imaging. 2014. PMID: 25060207 Free PMC article.
-
Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals.J Labelled Comp Radiopharm. 2014 Apr;57(4):224-30. doi: 10.1002/jlcr.3165. Epub 2013 Dec 18. J Labelled Comp Radiopharm. 2014. PMID: 24347474 Free PMC article. Review.
-
Cross-bridged macrocyclic chelators for stable complexation of copper radionuclides for PET imaging.Q J Nucl Med Mol Imaging. 2008 Jun;52(2):185-92. Epub 2007 Nov 28. Q J Nucl Med Mol Imaging. 2008. PMID: 18043536 Free PMC article. Review.
Cited by
-
Artificial Intelligence and the Future of Diagnostic and Therapeutic Radiopharmaceutical Development:: In Silico Smart Molecular Design.PET Clin. 2021 Oct;16(4):513-523. doi: 10.1016/j.cpet.2021.06.008. Epub 2021 Aug 5. PET Clin. 2021. PMID: 34364818 Free PMC article. Review.
-
Optimization of a Pretargeted Strategy for the PET Imaging of Colorectal Carcinoma via the Modulation of Radioligand Pharmacokinetics.Mol Pharm. 2015 Oct 5;12(10):3575-87. doi: 10.1021/acs.molpharmaceut.5b00294. Epub 2015 Aug 31. Mol Pharm. 2015. PMID: 26287993 Free PMC article.
-
Small Molecule Radiopharmaceuticals - A Review of Current Approaches.Front Med (Lausanne). 2016 Feb 23;3:5. doi: 10.3389/fmed.2016.00005. eCollection 2016. Front Med (Lausanne). 2016. PMID: 26942181 Free PMC article. Review.
-
Computer-Assisted Design of Peptide-Based Radiotracers.Int J Mol Sci. 2023 Apr 6;24(7):6856. doi: 10.3390/ijms24076856. Int J Mol Sci. 2023. PMID: 37047831 Free PMC article. Review.
-
Cu(I)-assisted click chemistry strategy for conjugation of non-protected cross-bridged macrocyclic chelators to tumour-targeting peptides.Dalton Trans. 2015 Mar 7;44(9):3945-8. doi: 10.1039/c4dt03897e. Dalton Trans. 2015. PMID: 25645688 Free PMC article.
References
-
- Paterson B. M.; Roselt P.; Denoyer D.; Cullinane C.; Binns D.; Noonan W.; Jeffery C. M.; Price R. I.; White J. M.; Hicks R. J.; Donnelly P. S. PET imaging of tumours with a 64Cu labeled macrobicyclic cage amine ligand tethered to Tyr3-octreotate. Dalton Trans. 2014, 43, 1386–1396. - PubMed
-
- Wadas T. J.; Anderson C. J. Radiolabeling of TETA- and CB-TE2A-conjugated peptides with copper-64. Nat. Protoc. 2007, 1, 3062–3068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

