An unstable Th epitope of P. falciparum fosters central memory T cells and anti-CS antibody responses
- PMID: 24983460
- PMCID: PMC4077652
- DOI: 10.1371/journal.pone.0100639
An unstable Th epitope of P. falciparum fosters central memory T cells and anti-CS antibody responses
Erratum in
- PLoS One. 2014;9(11):e113028. Yin, Liusong [added]
Abstract
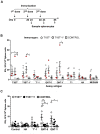
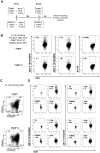
Malaria is transmitted by Plasmodium-infected anopheles mosquitoes. Widespread resistance of mosquitoes to insecticides and resistance of parasites to drugs highlight the urgent need for malaria vaccines. The most advanced malaria vaccines target sporozoites, the infective form of the parasite. A major target of the antibody response to sporozoites are the repeat epitopes of the circumsporozoite (CS) protein, which span almost one half of the protein. Antibodies to these repeats can neutralize sporozoite infectivity. Generation of protective antibody responses to the CS protein (anti-CS Ab) requires help by CD4 T cells. A CD4 T cell epitope from the CS protein designated T* was previously identified by screening T cells from volunteers immunized with irradiated P. falciparum sporozoites. The T* sequence spans twenty amino acids that contains multiple T cell epitopes restricted by various HLA alleles. Subunit malaria vaccines including T* are highly immunogenic in rodents, non-human primates and humans. In this study we characterized a highly conserved HLA-DRβ1*04:01 (DR4) restricted T cell epitope (QNT-5) located at the C-terminus of T*. We found that a peptide containing QNT-5 was able to elicit long-term anti-CS Ab responses and prime CD4 T cells in HLA-DR4 transgenic mice despite forming relatively unstable MHC-peptide complexes highly susceptible to HLA-DM editing. We attempted to improve the immunogenicity of QNT-5 by replacing the P1 anchor position with an optimal tyrosine residue. The modified peptide QNT-Y formed stable MHC-peptide complexes highly resistant to HLA-DM editing. Contrary to expectations, a linear peptide containing QNT-Y elicited almost 10-fold lower long-term antibody and IFN-γ responses compared to the linear peptide containing the wild type QNT-5 sequence. Some possibilities regarding why QNT-5 is more effective than QNT-Y in inducing long-term T cell and anti-CS Ab when used as vaccine are discussed.
Conflict of interest statement
Figures


Similar articles
-
A linear peptide containing minimal T- and B-cell epitopes of Plasmodium falciparum circumsporozoite protein elicits protection against transgenic sporozoite challenge.Infect Immun. 2006 Dec;74(12):6929-39. doi: 10.1128/IAI.01151-06. Epub 2006 Oct 9. Infect Immun. 2006. PMID: 17030584 Free PMC article.
-
A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types.J Immunol. 2001 Jan 1;166(1):481-9. doi: 10.4049/jimmunol.166.1.481. J Immunol. 2001. PMID: 11123327 Clinical Trial.
-
Plasmodium falciparum synthetic LbL microparticle vaccine elicits protective neutralizing antibody and parasite-specific cellular immune responses.Vaccine. 2013 Apr 8;31(15):1898-904. doi: 10.1016/j.vaccine.2013.02.027. Epub 2013 Feb 26. Vaccine. 2013. PMID: 23481177 Free PMC article.
-
T cell responses to repeat and non-repeat regions of the circumsporozoite protein detected in volunteers immunized with Plasmodium falciparum sporozoites.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:223-7. doi: 10.1590/s0074-02761992000700037. Mem Inst Oswaldo Cruz. 1992. PMID: 1364202 Review.
-
Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials.Parassitologia. 1999 Sep;41(1-3):397-402. Parassitologia. 1999. PMID: 10697892 Review.
Cited by
-
Progress in the Development of Subunit Vaccines against Malaria.Vaccines (Basel). 2020 Jul 10;8(3):373. doi: 10.3390/vaccines8030373. Vaccines (Basel). 2020. PMID: 32664421 Free PMC article. Review.
-
Distorted Immunodominance by Linker Sequences or other Epitopes from a Second Protein Antigen During Antigen-Processing.Sci Rep. 2017 Apr 19;7:46418. doi: 10.1038/srep46418. Sci Rep. 2017. PMID: 28422163 Free PMC article.
-
The putative role of Rhipicephalus microplus salivary serpins in the tick-host relationship.Insect Biochem Mol Biol. 2016 Apr;71:12-28. doi: 10.1016/j.ibmb.2016.01.004. Epub 2016 Feb 2. Insect Biochem Mol Biol. 2016. PMID: 26844868 Free PMC article.
References
-
- WHO (2011) WORLD HEALTH STATISTICS 2011. WHO Library Cataloguing-in-Publication Data.
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C (1967) Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 216: 160–162. - PubMed
-
- Clyde DF (1975) Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. The American journal of tropical medicine and hygiene 24: 397–401. - PubMed
-
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, et al. (2002) Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. The Journal of infectious diseases 185: 1155–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

