Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol
- PMID: 24984001
- PMCID: PMC4077692
- DOI: 10.1371/journal.pone.0100591
Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol
Abstract
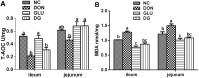
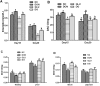
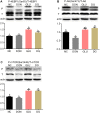
The mycotoxin deoxynivalenol (DON), one of the most common food contaminants, primarily targets the gastrointestinal tract to affect animal and human health. This study was conducted to examine the protective function of glutamic acid on intestinal injury and oxidative stress caused by DON in piglets. Twenty-eight piglets were assigned randomly into 4 dietary treatments (7 pigs/treatment): 1) uncontaminated control diet (NC), 2) NC+DON at 4 mg/kg (DON), 3) NC+2% glutamic acid (GLU), and 4) NC+2% glutamic acid + DON at 4 mg/kg (DG). At day 15, 30 and 37, blood samples were collected to determine serum concentrations of CAT (catalase), T-AOC (total antioxidant capacity), H2O2 (hydrogen peroxide), NO (nitric oxide), MDA (maleic dialdehyde), DAO (diamine oxidase) and D-lactate. Intestinal morphology, and the activation of Akt/mTOR/4EBP1 signal pathway, as well as the concentrations of H2O2, MDA, and DAO in kidney, liver and small intestine, were analyzed at day 37. Results showed that DON significantly (P<0.05) induced oxidative stress in piglets, while this stress was remarkably reduced with glutamic acid supplementation according to the change of oxidative parameters in blood and tissues. Meanwhile, DON caused obvious intestinal injury from microscopic observations and permeability indicators, which was alleviated by glutamic acid supplementation. Moreover, the inhibition of DON on Akt/mTOR/4EBP1 signal pathway was reduced by glutamic acid supplementation. Collectively, these data suggest that glutamic acid may be a useful nutritional regulator for DON-induced damage manifested as oxidative stress, intestinal injury and signaling inhibition.
Conflict of interest statement
Figures





References
-
- AE D (2006) Fusarium mycotoxins: Chemistry, genetics, and biology. St Paul: American Phytopathological Sciety.
-
- Eriksen GS PH (2004) Toxicological evaluation of trichothecenes in animal feed. Anim Feed Sci Technol 114: 205–239.
-
- Felicia W (2007) Measuring the economic impact of Fusarium toxins in animal feeds. Anim Feed Sci Technol 137: 363–374.
-
- Pestka JJ, Smolinski AT (2005) Deoxynivalenol: Toxicology and potential effects on humans. J Toxicol Env Heal B Vrit Rev 8: 39–69. - PubMed
-
- Kolf-Clauw M, Castellote J, Joly B, Bourges-Abella N, Raymond-Letron I, et al. (2009) Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: histopathological analysis. Toxicol in Vitro 23: 1580–1584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

