Functional architecture of the retina: development and disease
- PMID: 24984227
- PMCID: PMC4134977
- DOI: 10.1016/j.preteyeres.2014.06.003
Functional architecture of the retina: development and disease
Abstract
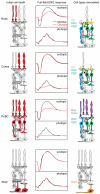
Structure and function are highly correlated in the vertebrate retina, a sensory tissue that is organized into cell layers with microcircuits working in parallel and together to encode visual information. All vertebrate retinas share a fundamental plan, comprising five major neuronal cell classes with cell body distributions and connectivity arranged in stereotypic patterns. Conserved features in retinal design have enabled detailed analysis and comparisons of structure, connectivity and function across species. Each species, however, can adopt structural and/or functional retinal specializations, implementing variations to the basic design in order to satisfy unique requirements in visual function. Recent advances in molecular tools, imaging and electrophysiological approaches have greatly facilitated identification of the cellular and molecular mechanisms that establish the fundamental organization of the retina and the specializations of its microcircuits during development. Here, we review advances in our understanding of how these mechanisms act to shape structure and function at the single cell level, to coordinate the assembly of cell populations, and to define their specific circuitry. We also highlight how structure is rearranged and function is disrupted in disease, and discuss current approaches to re-establish the intricate functional architecture of the retina.
Keywords: Mouse retina; Primate retina; Retinal cell mosaics; Retinal development; Retinal repair; Retinal synapses; Zebrafish retina.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



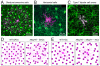
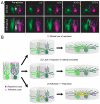
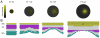







References
-
- Abbott CJ, Percival KA, Martin PR, Grunert U. Amacrine and bipolar inputs to midget and parasol ganglion cells in marmoset retina. Vis Neurosci. 2012;29:157–168. - PubMed
-
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi-light microscopic study of spectral connectivity. J Comp Neurol. 1994;343:387–405. - PubMed
-
- Alfano G, Conte I, Caramico T, Avellino R, Arno B, Pizzo MT, Tanimoto N, Beck SC, Huber G, Dolle P, Seeliger MW, Banfi S. Vax2 regulates retinoic acid distribution and cone opsin expression in the vertebrate eye. Development. 2011;138:261–271. - PubMed
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

