Protective role of frizzled-related protein B on matrix metalloproteinase induction in mouse chondrocytes
- PMID: 24984954
- PMCID: PMC4226985
- DOI: 10.1186/ar4599
Protective role of frizzled-related protein B on matrix metalloproteinase induction in mouse chondrocytes
Abstract
Introduction: Our objective was to investigate whether a lack of frizzled-related protein B (FrzB), an extracellular antagonist of the Wnt signaling pathways, could enhance cartilage degradation by facilitating the expression, release and activation of matrix metalloproteinases (MMPs) by chondrocytes in response to tissue-damaging stimuli.
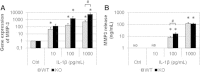
Methods: Cartilage explants from FrzB-/- and wild-type mice were challenged by excessive dynamic compression (0.5 Hz and 1 MPa for 6 hours). Load-induced glycosaminoglycan (GAG) release and MMP enzymatic activity were assessed. Interleukin-1β (IL-1β) (10, 100 and 1000 pg/mL for 24 hours) was used to stimulate primary cultures of articular chondrocytes from FrzB-/- and wild-type mice. The expression and release of MMP-3 and -13 were determined by RT-PCR, western blot and ELISA. The accumulation of β-catenin was assessed by RT-PCR and western blot.
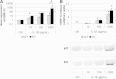
Results: Cartilage degradation, as revealed by a significant increase in GAG release (2.8-fold, P = 0.014) and MMP activity (4.5-fold, P = 0.014) by explants, was induced by an excessive load. Load-induced MMP activity appeared to be enhanced in FrzB-/- cartilage explants compared to wild-type (P = 0.17). IL-1β dose-dependently induced Mmp-13 and -3 gene expression and protein release by cultured chondrocytes. IL-1β-mediated increase in MMP-13 and -3 was slightly enhanced in FrzB-/- chondrocytes compared to wild-type (P = 0.05 and P = 0.10 at gene level, P = 0.17 and P = 0.10 at protein level, respectively). Analysis of Ctnn1b and Lef1 gene expression and β-catenin accumulation at protein level suggests that the enhanced catabolic response of FrzB-/- chondrocytes to IL-1β and load may be associated with an over-stimulation of the canonical Wnt/β-catenin pathway.
Conclusions: Our results suggest that FrzB may have a protective role on cartilage degradation and MMP induction in mouse chondrocytes by attenuating deleterious effects of the activation of the canonical Wnt/β-catenin pathway.
Figures


Similar articles
-
Tight regulation of wingless-type signaling in the articular cartilage - subchondral bone biomechanical unit: transcriptomics in Frzb-knockout mice.Arthritis Res Ther. 2012 Jan 20;14(1):R16. doi: 10.1186/ar3695. Arthritis Res Ther. 2012. PMID: 22264237 Free PMC article.
-
A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes.Arthritis Rheum. 2012 Aug;64(8):2589-600. doi: 10.1002/art.34425. Arthritis Rheum. 2012. PMID: 22328140
-
Stress-induced cartilage degradation does not depend on the NLRP3 inflammasome in human osteoarthritis and mouse models.Arthritis Rheum. 2012 Dec;64(12):3972-81. doi: 10.1002/art.34678. Arthritis Rheum. 2012. PMID: 22933232
-
GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition.Osteoarthritis Cartilage. 2014 Apr;22(4):566-77. doi: 10.1016/j.joca.2014.02.004. Epub 2014 Feb 19. Osteoarthritis Cartilage. 2014. PMID: 24561281
-
Wnt signalling in the articular cartilage: A matter of balance.Int J Exp Pathol. 2023 Apr;104(2):56-63. doi: 10.1111/iep.12472. Epub 2023 Feb 26. Int J Exp Pathol. 2023. PMID: 36843204 Free PMC article. Review.
Cited by
-
Emerging targets in osteoarthritis therapy.Curr Opin Pharmacol. 2015 Jun;22:51-63. doi: 10.1016/j.coph.2015.03.004. Epub 2015 Apr 10. Curr Opin Pharmacol. 2015. PMID: 25863583 Free PMC article. Review.
-
Nitric Oxide Mediates Crosstalk between Interleukin 1β and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression.Int J Mol Sci. 2017 Nov 22;18(11):2491. doi: 10.3390/ijms18112491. Int J Mol Sci. 2017. PMID: 29165387 Free PMC article.
-
Exploring the Crosstalk between Hydrostatic Pressure and Adipokines: An In Vitro Study on Human Osteoarthritic Chondrocytes.Int J Mol Sci. 2021 Mar 9;22(5):2745. doi: 10.3390/ijms22052745. Int J Mol Sci. 2021. PMID: 33803113 Free PMC article.
-
WTAP-mediated m6A modification of FRZB triggers the inflammatory response via the Wnt signaling pathway in osteoarthritis.Exp Mol Med. 2024 Feb;56(1):156-167. doi: 10.1038/s12276-023-01135-5. Epub 2024 Jan 4. Exp Mol Med. 2024. PMID: 38172596 Free PMC article.
-
Characterization of osteoarthritic human knees indicates potential sex differences.Biol Sex Differ. 2016 Jun 2;7:27. doi: 10.1186/s13293-016-0080-z. eCollection 2016. Biol Sex Differ. 2016. PMID: 27257472 Free PMC article.
References
-
- Dell’accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 2008;58:1410–1421. - PubMed
-
- Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. - PubMed
-
- Papathanasiou I, Malizos KN, Tsezou A. Bone morphogenetic protein-2-induced Wnt/beta-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes. Arthritis Res Ther. 2012;14:R82. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

