Germ cell transport across the seminiferous epithelium during spermatogenesis
- PMID: 24985332
- PMCID: PMC4103058
- DOI: 10.1152/physiol.00001.2014
Germ cell transport across the seminiferous epithelium during spermatogenesis
Abstract
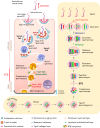
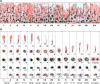
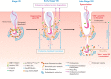
Transport of germ cells across the seminiferous epithelium is crucial to spermatogenesis. Its disruption causes infertility. Signaling molecules, such as focal adhesion kinase, c-Yes, c-Src, and intercellular adhesion molecules 1 and 2, are involved in these events by regulating actin-based cytoskeleton via their action on actin-regulating proteins, endocytic vesicle-mediated protein trafficking, and adhesion protein complexes. We critically evaluate these findings and provide a hypothetical framework that regulates these events.
©2014 Int. Union Physiol. Sci./Am. Physiol. Soc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures

References
-
- Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl 29: 469–487, 2008 - PubMed
-
- Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol 124: 211–215, 1980 - PubMed
-
- Amann RP, Schanbacher BD. Physiology of male reproduction. J Anim Sci 57, Suppl 2: 380–403, 1983 - PubMed
-
- Auharek SA, Avelar GF, Lara NLM, Sharpe RM, Franca LR. Sertoli cell numbers and spermatogenic efficency are increased in inducible nitric oxide synthase (iNOS) mutant-mice. Int J Androl 34: e621–e629, 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

