Effects of repeated intrathecal triamcinolone-acetonide application on cerebrospinal fluid biomarkers of axonal damage and glial activity in multiple sclerosis patients
- PMID: 24986188
- PMCID: PMC4245486
- DOI: 10.1007/s40291-014-0114-3
Effects of repeated intrathecal triamcinolone-acetonide application on cerebrospinal fluid biomarkers of axonal damage and glial activity in multiple sclerosis patients
Abstract
Background and objectives: Multiple sclerosis (MS) is the most common inflammatory disease of the central nervous system in young adults. Over time, the disease progresses and, with accumulating disability, symptoms such as spasticity may occur. Although several treatment options are available, some patients may not respond to first-line therapeutics. However, some of these patients may benefit from intrathecally administered triamcinolone-acetonide (TCA), a derivative of glucocorticosteroids (GCS). GCS may have neurotoxic effects, and cell apoptosis may occur. The aim of this study was to investigate the effects of TCA on biomarkers in the cerebrospinal fluid (CSF) suggestive of neurodegeneration.
Methods: In order to assess neurotoxic effects of TCA, neurofilament heavy-chain (NfH)(SMI35), tau protein, and S-100B protein levels were determined before and during treatment with TCA in 54 patients with primary progressive MS, as well as relapsing MS (relapsing-remitting and secondary progressive MS).
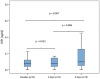
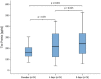
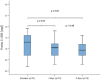
Results: NfH(SMI35) levels in the CSF of patients treated with TCA intrathecally did not increase significantly during the treatment cycle (p = 0.068). After application of TCA, tau protein levels were increased significantly at day 4 (p = 0.03) and at day 8 (p ≤ 0.001). S-100B protein levels decreased significantly (p ≤ 0.05) during treatment with TCA.
Conclusion: NfH(SMI35) levels did not change significantly; however, tau protein levels did increase significantly within the reference range. Taking these findings together, the long-term effects of TCA on NfH(SMI35) and tau protein levels need to be investigated further to understand whether levels of both biomarkers will change over repeated TCA applications. Interestingly, S-100B protein levels decreased significantly during the first applications, which may have represented reduced astrocytic activity during TCA treatment.
Figures
Similar articles
-
Glial and neuroaxonal biomarkers in a multiple sclerosis (MS) cohort.Hell J Nucl Med. 2019 Sep-Dec;22 Suppl 2:113-121. Hell J Nucl Med. 2019. PMID: 31802051
-
Axonal damage accumulates in the progressive phase of multiple sclerosis: three year follow up study.J Neurol Neurosurg Psychiatry. 2005 Feb;76(2):206-11. doi: 10.1136/jnnp.2004.043315. J Neurol Neurosurg Psychiatry. 2005. PMID: 15654034 Free PMC article.
-
Repeat intrathecal triamcinolone acetonide application is beneficial in progressive MS patients.Eur J Neurol. 2006 Jan;13(1):72-6. doi: 10.1111/j.1468-1331.2006.01145.x. Eur J Neurol. 2006. PMID: 16420395 Clinical Trial.
-
Management of spasticity in progressive multiple sclerosis: efficacy of repeated intrathecal triamcinolone acetonide administration.Curr Pharm Des. 2012;18(29):4564-9. doi: 10.2174/138161212802502251. Curr Pharm Des. 2012. PMID: 22612755 Review.
-
Neurofilament light chain in the assessment of patients with multiple sclerosis.Arq Neuropsiquiatr. 2019 Jul 15;77(6):436-441. doi: 10.1590/0004-282X20190060. Arq Neuropsiquiatr. 2019. PMID: 31314847 Review.
Cited by
-
Intrathecal triamcinolone acetonide exerts anti-inflammatory effects on Lewis rat experimental autoimmune neuritis and direct anti-oxidative effects on Schwann cells.J Neuroinflammation. 2019 Mar 9;16(1):58. doi: 10.1186/s12974-019-1445-0. J Neuroinflammation. 2019. PMID: 30851725 Free PMC article.
-
Predictors for Therapy Response to Intrathecal Corticosteroid Therapy in Multiple Sclerosis.Front Neurol. 2019 Feb 22;10:132. doi: 10.3389/fneur.2019.00132. eCollection 2019. Front Neurol. 2019. PMID: 30853935 Free PMC article.
-
Neurofilaments in progressive multiple sclerosis: a systematic review.J Neurol. 2021 Sep;268(9):3212-3222. doi: 10.1007/s00415-020-09917-x. Epub 2020 May 23. J Neurol. 2021. PMID: 32447549 Free PMC article.
-
Recent Progress in the Identification of Early Transition Biomarkers from Relapsing-Remitting to Progressive Multiple Sclerosis.Int J Mol Sci. 2023 Feb 22;24(5):4375. doi: 10.3390/ijms24054375. Int J Mol Sci. 2023. PMID: 36901807 Free PMC article. Review.
-
Systemic Effects by Intrathecal Administration of Triamcinolone Acetonide in Patients With Multiple Sclerosis.Front Endocrinol (Lausanne). 2020 Aug 27;11:574. doi: 10.3389/fendo.2020.00574. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32982971 Free PMC article.
References
-
- National Multiple Sclerosis Society. Multiple sclerosis FAQs. http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-a.... Accessed 7 Aug 2013.
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–911. doi: 10.1212/WNL.46.4.907. - DOI - PubMed
-
- Svensson J, Borg S, Nilsson P. Costs and quality of life in multiple sclerosis patients with spasticity. Acta Neurol Scand. 2014 Jan;129(1):13–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

