Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71
- PMID: 24986489
- PMCID: PMC4145081
- DOI: 10.1007/s13238-014-0087-3
Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71
Abstract
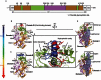
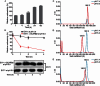
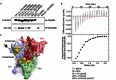
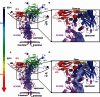
Unlike the well-established picture for the entry of enveloped viruses, the mechanism of cellular entry of non-enveloped eukaryotic viruses remains largely mysterious. Picornaviruses are representative models for such viruses, and initiate this entry process by their functional receptors. Here we present the structural and functional studies of SCARB2, a functional receptor of the important human enterovirus 71 (EV71). SCARB2 is responsible for attachment as well as uncoating of EV71. Differences in the structures of SCARB2 under neutral and acidic conditions reveal that SCARB2 undergoes a pivotal pH-dependent conformational change which opens a lipid-transfer tunnel to mediate the expulsion of a hydrophobic pocket factor from the virion, a pre-requisite for uncoating. We have also identified the key residues essential for attachment to SCARB2, identifying the canyon region of EV71 as mediating the receptor interaction. Together these results provide a clear understanding of cellular attachment and initiation of uncoating for enteroviruses.
Figures

Comment in
-
The cellular receptor for enterovirus 71.Protein Cell. 2014 Sep;5(9):655-7. doi: 10.1007/s13238-014-0092-6. Protein Cell. 2014. PMID: 25103897 Free PMC article. No abstract available.
References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Bergelson JM, Coyne CB (2013) Picornavirus entry. In: Viral entry into host cells. Springer, New York, pp 24–41
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

