CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers
- PMID: 24986516
- PMCID: PMC4122288
- DOI: 10.1158/0008-5472.CAN-13-2923
CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers
Abstract
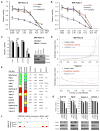
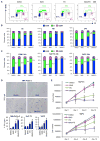
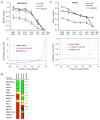
Loss-of-function mutations in p16(INK4A) (CDKN2A) occur in approximately 80% of sporadic pancreatic ductal adenocarcinoma (PDAC), contributing to its early progression. Although this loss activates the cell-cycle-dependent kinases CDK4/6, which have been considered as drug targets for many years, p16(INK4A)-deficient PDAC cells are inherently resistant to CDK4/6 inhibitors. This study searched for targeted therapies that might synergize with CDK4/6 inhibition in this setting. We report that the IGF1R/IR inhibitor BMS-754807 cooperated with the CDK4/6 inhibitor PD-0332991 to strongly block proliferation of p16(INK4A)-deficient PDAC cells in vitro and in vivo. Sensitivity to this drug combination correlated with reduced activity of the master cell growth regulator mTORC1. Accordingly, replacing the IGF1R/IR inhibitor with the rapalog inhibitor temsirolimus broadened the sensitivity of PDAC cells to CDK4/6 inhibition. Our results establish targeted therapy combinations with robust cytostatic activity in p16(INK4A)-deficient PDAC cells and possible implications for improving treatment of a broad spectrum of human cancers characterized by p16(INK4A) loss.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

