The outcome of B-cell receptor signaling in chronic lymphocytic leukemia: proliferation or anergy
- PMID: 24986876
- PMCID: PMC4077074
- DOI: 10.3324/haematol.2013.098384
The outcome of B-cell receptor signaling in chronic lymphocytic leukemia: proliferation or anergy
Abstract
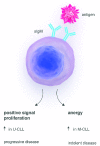
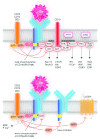
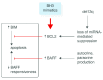
Biologists and clinicians agree that the B-cell receptor influences the behavior of chronic lymphocytic leukemia, and promising new drugs are aimed at receptor-associated kinases. Engagement of surface immunoglobulin by antigen is a key driver of malignant cells with outcome influenced by the nature of the cell, the level of stimulation and the microenvironment. Analysis of surface immunoglobulin-mediated signaling in the two major disease subsets defined by IGHV mutational status reveals bifurcation of responses toward proliferation or anergy. Mutated chronic lymphocytic leukemia, generally of relatively good prognosis, is mainly, but not exclusively, driven towards anergy in vivo. In contrast, unmutated chronic lymphocytic leukemia shows less evidence for anergy in vivo retaining more responsiveness to surface immunoglobulin M-mediated signaling, possibly explaining increased tumor progression. Expression and function of surface immunoglobulin M in unmutated chronic lymphocytic leukemia appear rather homogeneous, but mutated chronic lymphocytic leukemia exhibits a highly heterogeneous profile that may relate to further variable clinical behavior within this subset. Anergy should increase susceptibility to apoptosis but, in leukemic cells, this may be countered by overexpression of the B-cell lymphoma-2 survival protein. Maintained anergy spreads to chemokines and adhesion molecules, restraining homing and migration. However, anergy is not necessarily completely benign, being able to reverse and regenerate surface immunoglobulin M-mediated responses. A two-pronged attack on proliferative and anti-apoptotic pathways may succeed. Increased understanding of how chronic lymphocytic leukemia cells are driven to anergy or proliferation should reveal predictive biomarkers of progression and of likely response to kinase inhibitors, which could assist therapeutic decisions.
Copyright© Ferrata Storti Foundation.
Figures


References
-
- Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005; 5(4):251–62 - PubMed
-
- Stevenson FK, Stevenson GT. Follicular lymphoma and the immune system: from pathogenesis to antibody therapy. Blood. 2012;119(16):3659–67 - PubMed
-
- Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011; 118(16):4313–20 - PubMed
-
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54 - PubMed
-
- Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

