In vitro reconstitution of a highly processive recombinant human dynein complex
- PMID: 24986880
- PMCID: PMC4158905
- DOI: 10.15252/embj.201488792
In vitro reconstitution of a highly processive recombinant human dynein complex
Abstract
Cytoplasmic dynein is an approximately 1.4 MDa multi-protein complex that transports many cellular cargoes towards the minus ends of microtubules. Several in vitro studies of mammalian dynein have suggested that individual motors are not robustly processive, raising questions about how dynein-associated cargoes can move over long distances in cells. Here, we report the production of a fully recombinant human dynein complex from a single baculovirus in insect cells. Individual complexes very rarely show directional movement in vitro. However, addition of dynactin together with the N-terminal region of the cargo adaptor BICD2 (BICD2N) gives rise to unidirectional dynein movement over remarkably long distances. Single-molecule fluorescence microscopy provides evidence that BICD2N and dynactin stimulate processivity by regulating individual dynein complexes, rather than by promoting oligomerisation of the motor complex. Negative stain electron microscopy reveals the dynein-dynactin-BICD2N complex to be well ordered, with dynactin positioned approximately along the length of the dynein tail. Collectively, our results provide insight into a novel mechanism for coordinating cargo binding with long-distance motor movement.
Keywords: Bicaudal‐D; dynactin; dynein; microtubules; processivity.
© 2014 MRC Laboratory of Molecular Biology. Published under the terms of the CC BY 4.0 license.
Figures
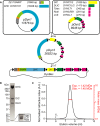
Schematic overview of the dynein genes present in the pDyn1 and pDyn2 plasmids and the assembly of pDyn3 using Cre recombinase. pDyn3 was subsequently integrated into the baculoviral genome by Tn7 transposition to form DynBac. T indicates a Tobacco Etch Virus (TEV) protease cleavage site; black triangles and black rectangles represent PolH promoter and SV40 terminator sequences, respectively. Not to scale.
Coomassie-stained SDS–PAGE gel of purified recombinant dynein complex. Inset is the 10–15 kDa range from a gel with better low-molecular-weight separation on which bands corresponding to the different light chains can be discriminated.
SEC-MALS of recombinant dynein. Mean observed molar mass (Obs.) and expected (Exp) molar mass are indicated. Expected molar mass was calculated for a dimeric complex of the DHC, DIC, DLIC, Tctex, Robl and LC8 chains. V0 indicates the void volume of the column.
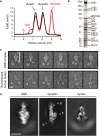
Size-exclusion chromatography traces for a mixture of dynein and dynactin alone (black trace; 1 dynein complex to 2 dynactin complexes) and dynein, dynactin and BICDN (red trace; 1 dynein complex to 2 dynactin complexes to 20 BICD2N dimers). DDB, dynein–dynactin–BICD2N complex. V0 indicates the void volume of the column.
SYPRO Ruby-stained SDS–PAGE gel of the pooled and concentrated fractions collected from the DDB peak in (A). In addition to dynein subunits and BICD2N, multiple bands corresponding to dynactin subunits are observed. p135 is an spliceoform of p150 (Tokito et al, 1996). Note that BICD2N has a predicted molecular mass of 72.4 kDa due to the presence of the GFP tag.
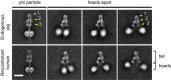
Representative negative stain EM single particles (low-pass filtered to 30 Å) of the DDB complex and recombinant human dynein. Note the significantly larger tail domain of the DDB complex (white bracket) and the range of head positions for both complexes. Scale bar, 20 nm.
2D class average of the DDB tail compared to 2D class averages of dynactin and the recombinant human dynein tail. Alignment of the dynein and DDB tails was performed by applying a binary mask that excluded the flexible dynein heads to all particles (see Supplementary Fig S2 and Materials and Methods). This procedure results in the head domains appearing as a blur following removal of the mask. Dynactin structural features are labelled as follows: p, pointed end; s, shoulder/projecting arm; b, barbed end. The dashed lines allow a size comparison of the DDB tail domain to the dynein tail and dynactin alone. Dynactin appears to be positioned approximately along the length of dynein tail domain in the DDB complex. The positions of the pointed end, shoulder/projecting arm and barbed end cannot be unambiguously determined in the class average of the DDB tail. Scale bar, 20 nm.
Comment in
-
Traffic control: adaptor proteins guide dynein-cargo takeoff.EMBO J. 2014 Sep 1;33(17):1845-6. doi: 10.15252/embj.201489450. Epub 2014 Jul 24. EMBO J. 2014. PMID: 25061224 Free PMC article.
-
Backseat drivers: Regulation of dynein motility.Cell Res. 2014 Dec;24(12):1385-6. doi: 10.1038/cr.2014.115. Epub 2014 Aug 22. Cell Res. 2014. PMID: 25145357 Free PMC article.
-
Sorting out microtubule-based transport.Nat Rev Mol Cell Biol. 2021 Feb;22(2):73. doi: 10.1038/s41580-020-00320-y. Nat Rev Mol Cell Biol. 2021. PMID: 33288890 No abstract available.
References
-
- Allan VJ. Cytoplasmic dynein. Biochem Soc Trans. 2011;39:1169–1178. - PubMed
-
- Amos LA. Brain dynein crossbridges microtubules into bundles. J Cell Sci. 1989;93(Pt 1):19–28. - PubMed
-
- Bieling P, Telley IA, Hentrich C, Piehler J, Surrey T. Fluorescence microscopy assays on chemically functionalized surfaces for quantitative imaging of microtubule, motor, and +TIP dynamics. Methods Cell Biol. 2010;95:555–580. - PubMed
-
- Carter AP. Crystal clear insights into how the dynein motor moves. J Cell Sci. 2013;126:705–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

