A population-based study of KCNH7 p.Arg394His and bipolar spectrum disorder
- PMID: 24986916
- PMCID: PMC4222358
- DOI: 10.1093/hmg/ddu335
A population-based study of KCNH7 p.Arg394His and bipolar spectrum disorder
Abstract
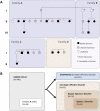
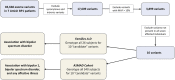
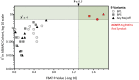
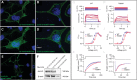
We conducted blinded psychiatric assessments of 26 Amish subjects (52 ± 11 years) from four families with prevalent bipolar spectrum disorder, identified 10 potentially pathogenic alleles by exome sequencing, tested association of these alleles with clinical diagnoses in the larger Amish Study of Major Affective Disorder (ASMAD) cohort, and studied mutant potassium channels in neurons. Fourteen of 26 Amish had bipolar spectrum disorder. The only candidate allele shared among them was rs78247304, a non-synonymous variant of KCNH7 (c.1181G>A, p.Arg394His). KCNH7 c.1181G>A and nine other potentially pathogenic variants were subsequently tested within the ASMAD cohort, which consisted of 340 subjects grouped into controls subjects and affected subjects from overlapping clinical categories (bipolar 1 disorder, bipolar spectrum disorder and any major affective disorder). KCNH7 c.1181G>A had the highest enrichment among individuals with bipolar spectrum disorder (χ(2) = 7.3) and the strongest family-based association with bipolar 1 (P = 0.021), bipolar spectrum (P = 0.031) and any major affective disorder (P = 0.016). In vitro, the p.Arg394His substitution allowed normal expression, trafficking, assembly and localization of HERG3/Kv11.3 channels, but altered the steady-state voltage dependence and kinetics of activation in neuronal cells. Although our genome-wide statistical results do not alone prove association, cumulative evidence from multiple independent sources (parallel genome-wide study cohorts, pharmacological studies of HERG-type potassium channels, electrophysiological data) implicates neuronal HERG3/Kv11.3 potassium channels in the pathophysiology of bipolar spectrum disorder. Such a finding, if corroborated by future studies, has implications for mental health services among the Amish, as well as development of drugs that specifically target HERG3/Kv11.3.
© The Author 2014. Published by Oxford University Press.
Figures
References
-
- World Health Organization. 2008. mhGAP: Mental Health Gap Action Programme: scaling up care for mental, neurological and substance use disorders http://www.who.int/mental_health/mhgap/en/ .
-
- Hurvitz K., Kandi D. QuickStats: suicide and homicide rates, by age group—United States, 2009. MMWR. 2012;61:543.
-
- McNamara R.K., Nandagopal J.J., Strakowski S.M., DelBello M.P. Preventative strategies for early-onset bipolar disorder: towards a clinical staging model. CNS Drugs. 2010;24:983–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

