Interstitial cells: regulators of smooth muscle function
- PMID: 24987007
- PMCID: PMC4152167
- DOI: 10.1152/physrev.00037.2013
Interstitial cells: regulators of smooth muscle function
Abstract
Smooth muscles are complex tissues containing a variety of cells in addition to muscle cells. Interstitial cells of mesenchymal origin interact with and form electrical connectivity with smooth muscle cells in many organs, and these cells provide important regulatory functions. For example, in the gastrointestinal tract, interstitial cells of Cajal (ICC) and PDGFRα(+) cells have been described, in detail, and represent distinct classes of cells with unique ultrastructure, molecular phenotypes, and functions. Smooth muscle cells are electrically coupled to ICC and PDGFRα(+) cells, forming an integrated unit called the SIP syncytium. SIP cells express a variety of receptors and ion channels, and conductance changes in any type of SIP cell affect the excitability and responses of the syncytium. SIP cells are known to provide pacemaker activity, propagation pathways for slow waves, transduction of inputs from motor neurons, and mechanosensitivity. Loss of interstitial cells has been associated with motor disorders of the gut. Interstitial cells are also found in a variety of other smooth muscles; however, in most cases, the physiological and pathophysiological roles for these cells have not been clearly defined. This review describes structural, functional, and molecular features of interstitial cells and discusses their contributions in determining the behaviors of smooth muscle tissues.
Copyright © 2014 the American Physiological Society.
Figures



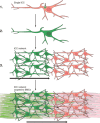
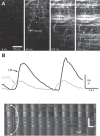
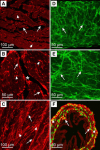
Comment in
-
Concerning the validity of gastrointestinal extracellular recordings.Physiol Rev. 2015 Apr;95(2):691-2. doi: 10.1152/physrev.00005.2015. Physiol Rev. 2015. PMID: 25834235 No abstract available.
-
Reply to O'Grady et al.Physiol Rev. 2015 Apr;95(2):693-4. doi: 10.1152/physrev.00006.2015. Physiol Rev. 2015. PMID: 25834236 No abstract available.
References
-
- Abe H. The mammalian oviductal epithelium: regional variations in cytological and functional aspects of the oviductal secretory cells. Histol Histopathol 11: 743–768, 1996 - PubMed
-
- Adams CE. Rate of sperm transport in the female reproductive tract of the rabbit. J Endocrinol 13: xxi–xxii, 1956 - PubMed
-
- Alberti E, Mikkelsen HB, Wang XY, Diaz M, Larsen JO, Huizinga JD, Jimenez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol 292: G1499–G1510, 2007 - PubMed
-
- Allix S, Reyes-Gomez E, Aubin-Houzelstein G, Noel D, Tiret L, Panthier JJ, Bernex F. Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence. Biol Reprod 79: 510–517, 2008 - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DK57236/DK/NIDDK NIH HHS/United States
- R37 DK040569/DK/NIDDK NIH HHS/United States
- P20 GM103513/GM/NIGMS NIH HHS/United States
- DK078736/DK/NIDDK NIH HHS/United States
- R01 DK098388/DK/NIDDK NIH HHS/United States
- R01 DK98388/DK/NIDDK NIH HHS/United States
- P01 DK41315/DK/NIDDK NIH HHS/United States
- R01 DK91336/DK/NIDDK NIH HHS/United States
- R01 DK057236/DK/NIDDK NIH HHS/United States
- P01 DK041315/DK/NIDDK NIH HHS/United States
- R01 DK091336/DK/NIDDK NIH HHS/United States
- R01 DK078736/DK/NIDDK NIH HHS/United States
- P30 GM110767/GM/NIGMS NIH HHS/United States
- R37 DK40569/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

