Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare
- PMID: 24987092
- PMCID: PMC4161246
- DOI: 10.1128/mBio.01364-14
Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare
Abstract
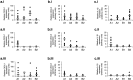
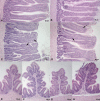
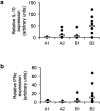
Campylobacter jejuni is the leading cause of bacterial food-borne infection; chicken meat is its main source. C. jejuni is considered commensal in chickens based on experimental models unrepresentative of commercial production. Here we show that the paradigm of Campylobacter commensalism in the chicken is flawed. Through experimental infection of four commercial breeds of broiler chickens, we show that breed has a significant effect on C. jejuni infection and the immune response of the animals, although these factors have limited impact on the number of bacteria in chicken ceca. All breeds mounted an innate immune response. In some breeds, this response declined when interleukin-10 was expressed, consistent with regulation of the intestinal inflammatory response, and these birds remained healthy. In another breed, there was a prolonged inflammatory response, evidence of damage to gut mucosa, and diarrhea. We show that bird type has a major impact on infection biology of C. jejuni. In some breeds, infection leads to disease, and the bacterium cannot be considered a harmless commensal. These findings have implications for the welfare of chickens in commercial production where C. jejuni infection is a persistent problem. Importance: Campylobacter jejuni is the most common cause of food-borne bacterial diarrheal disease in the developed world. Chicken is the most common source of infection. C. jejuni infection of chickens had previously not been considered to cause disease, and it was thought that C. jejuni was part of the normal microbiota of birds. In this work, we show that modern rapidly growing chicken breeds used in intensive production systems have a strong inflammatory response to C. jejuni infection that can lead to diarrhea, which, in turn, leads to damage to the feet and legs on the birds due to standing on wet litter. The response and level of disease varied between breeds and is related to regulation of the inflammatory immune response. These findings challenge the paradigm that C. jejuni is a harmless commensal of chickens and that C. jejuni infection may have substantial impact on animal health and welfare in intensive poultry production:
Copyright © 2014 Humphrey et al.
Figures

References
-
- European Food Safety Authority 2010. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008 - Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. Avgust:1503–1602. 10.2903/j.efsa.2010.1503 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

