Engineered endolysin-based "Artilysins" to combat multidrug-resistant gram-negative pathogens
- PMID: 24987094
- PMCID: PMC4161244
- DOI: 10.1128/mBio.01379-14
Engineered endolysin-based "Artilysins" to combat multidrug-resistant gram-negative pathogens
Abstract
The global threat to public health posed by emerging multidrug-resistant bacteria in the past few years necessitates the development of novel approaches to combat bacterial infections. Endolysins encoded by bacterial viruses (or phages) represent one promising avenue of investigation. These enzyme-based antibacterials efficiently kill Gram-positive bacteria upon contact by specific cell wall hydrolysis. However, a major hurdle in their exploitation as antibacterials against Gram-negative pathogens is the impermeable lipopolysaccharide layer surrounding their cell wall. Therefore, we developed and optimized an approach to engineer these enzymes as outer membrane-penetrating endolysins (Artilysins), rendering them highly bactericidal against Gram-negative pathogens, including Pseudomonas aeruginosa and Acinetobacter baumannii. Artilysins combining a polycationic nonapeptide and a modular endolysin are able to kill these (multidrug-resistant) strains in vitro with a 4 to 5 log reduction within 30 min. We show that the activity of Artilysins can be further enhanced by the presence of a linker of increasing length between the peptide and endolysin or by a combination of both polycationic and hydrophobic/amphipathic peptides. Time-lapse microscopy confirmed the mode of action of polycationic Artilysins, showing that they pass the outer membrane to degrade the peptidoglycan with subsequent cell lysis. Artilysins are effective in vitro (human keratinocytes) and in vivo (Caenorhabditis elegans). Importance: Bacterial resistance to most commonly used antibiotics is a major challenge of the 21st century. Infections that cannot be treated by first-line antibiotics lead to increasing morbidity and mortality, while millions of dollars are spent each year by health care systems in trying to control antibiotic-resistant bacteria and to prevent cross-transmission of resistance. Endolysins--enzymes derived from bacterial viruses--represent a completely novel, promising class of antibacterials based on cell wall hydrolysis. Specifically, they are active against Gram-positive species, which lack a protective outer membrane and which have a low probability of resistance development. We modified endolysins by protein engineering to create Artilysins that are able to pass the outer membrane and become active against Pseudomonas aeruginosa and Acinetobacter baumannii, two of the most hazardous drug-resistant Gram-negative pathogens.
Copyright © 2014 Briers et al.
Figures
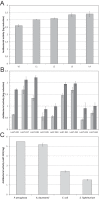
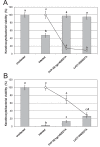
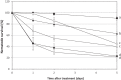
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
