Structure, function, and plasticity of GABA transporters
- PMID: 24987330
- PMCID: PMC4060055
- DOI: 10.3389/fncel.2014.00161
Structure, function, and plasticity of GABA transporters
Abstract
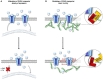
GABA transporters belong to a large family of neurotransmitter:sodium symporters. They are widely expressed throughout the brain, with different levels of expression in different brain regions. GABA transporters are present in neurons and in astrocytes and their activity is crucial to regulate the extracellular concentration of GABA under basal conditions and during ongoing synaptic events. Numerous efforts have been devoted to determine the structural and functional properties of GABA transporters. There is also evidence that the expression of GABA transporters on the cell membrane and their lateral mobility can be modulated by different intracellular signaling cascades. The strength of individual synaptic contacts and the activity of entire neuronal networks may be finely tuned by altering the density, distribution and diffusion rate of GABA transporters within the cell membrane. These findings are intriguing because they suggest the existence of complex regulatory systems that control the plasticity of GABAergic transmission in the brain. Here we review the current knowledge on the structural and functional properties of GABA transporters and highlight the molecular mechanisms that alter the expression and mobility of GABA transporters at central synapses.
Keywords: GABA; GABA transporters; GAT1; GAT3; SLC6; synaptic plasticity; synaptic transmission; uptake.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

