The impact of flow-induced forces on the morphogenesis of the outflow tract
- PMID: 24987377
- PMCID: PMC4060072
- DOI: 10.3389/fphys.2014.00225
The impact of flow-induced forces on the morphogenesis of the outflow tract
Abstract
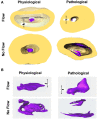
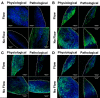
One percent of infants are born with congenital heart disease (CHD), which commonly involves outflow tract (OFT) defects. These infants often require complex surgeries, which are associated with long term adverse remodeling effects, and receive replacement valves with limited strength, biocompatibility, and growth capability. To address these problematic issues, researchers have carried out investigations in valve development and valve mechanics. A longstanding hypothesis is that flow-induced forces regulate fibrous valve development, however, the specific mechanisms behind this mechanotransduction remain unclear. The purpose of this study was to implement an in vitro system of outflow tract development to test the response of embryonic OFT tissues to fluid flow. A dynamic, three-dimensional bioreactor system was used to culture embryonic OFT tissue under different levels of flow as well as the absence of flow. In the absence of flow, OFT tissues took on a more primitive phenotype that is characteristic of early OFT cushion development where widely dispersed mesenchymal cells are surrounded by a sparse, disorganized extracellular matrix (ECM). Whereas OFT tissues subjected to physiologically matched flow formed compact mounds of cells, initated, fibrous ECM development, while prolonged supraphysiological flow resulted in abnormal tissue remodeling. This study indicates that both the timing and magnitude of flow alter cellular processes that determine if OFT precursor tissue undergoes normal or pathological development. Specifically, these experiments showed that flow-generated forces regulate the deposition and localization of fibrous ECM proteins, indicating that mechanosensitive signaling pathways are capable of driving pathological OFT development if flows are not ideal.
Keywords: bioreactor; fibrotic development; hemodynamics; mechanotransduction; outflow tract.
Figures









Similar articles
-
Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves.Dev Biol. 2013 Feb 15;374(2):345-56. doi: 10.1016/j.ydbio.2012.11.023. Epub 2012 Dec 19. Dev Biol. 2013. PMID: 23261934
-
Altered Hemodynamics in the Embryonic Heart Affects Outflow Valve Development.J Cardiovasc Dev Dis. 2015;2(2):108-124. doi: 10.3390/jcdd2020108. Epub 2015 May 15. J Cardiovasc Dev Dis. 2015. PMID: 26878022 Free PMC article.
-
Fluid dynamics and forces in the HH25 avian embryonic outflow tract.Biomech Model Mechanobiol. 2019 Aug;18(4):1123-1137. doi: 10.1007/s10237-019-01132-6. Epub 2019 Feb 27. Biomech Model Mechanobiol. 2019. PMID: 30810888
-
Outflow Tract Formation-Embryonic Origins of Conotruncal Congenital Heart Disease.J Cardiovasc Dev Dis. 2021 Apr 9;8(4):42. doi: 10.3390/jcdd8040042. J Cardiovasc Dev Dis. 2021. PMID: 33918884 Free PMC article. Review.
-
Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways.Mol Genet Metab. 2003 Sep-Oct;80(1-2):27-35. doi: 10.1016/j.ymgme.2003.07.004. Mol Genet Metab. 2003. PMID: 14567955 Review.
Cited by
-
Editorial: Mechanotransduction and development of cardiovascular form and function.Front Physiol. 2015 Apr 28;6:131. doi: 10.3389/fphys.2015.00131. eCollection 2015. Front Physiol. 2015. PMID: 25972813 Free PMC article. No abstract available.
-
Organ Dynamics and Hemodynamic of the Whole HH25 Avian Embryonic Heart, Revealed by Ultrasound Biomicroscopy, Boundary Tracking, and Flow Simulations.Sci Rep. 2019 Dec 2;9(1):18072. doi: 10.1038/s41598-019-54061-w. Sci Rep. 2019. PMID: 31792224 Free PMC article.
-
Animal Models for Heart Valve Research and Development.Drug Discov Today Dis Models. 2017 Summer;24:55-62. doi: 10.1016/j.ddmod.2018.04.001. Epub 2018 May 28. Drug Discov Today Dis Models. 2017. PMID: 30631375 Free PMC article.
-
Influence of blood flow on cardiac development.Prog Biophys Mol Biol. 2018 Sep;137:95-110. doi: 10.1016/j.pbiomolbio.2018.05.005. Epub 2018 May 24. Prog Biophys Mol Biol. 2018. PMID: 29772208 Free PMC article. Review.
-
A Novel Ex Ovo Banding Technique to Alter Intracardiac Hemodynamics in an Embryonic Chicken System.J Vis Exp. 2016 May 13;(111):53955. doi: 10.3791/53955. J Vis Exp. 2016. PMID: 27213265 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

