Coronavirus infection, ER stress, apoptosis and innate immunity
- PMID: 24987391
- PMCID: PMC4060729
- DOI: 10.3389/fmicb.2014.00296
Coronavirus infection, ER stress, apoptosis and innate immunity
Abstract
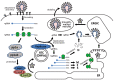
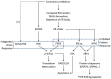
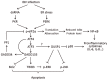
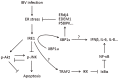
The replication of coronavirus, a family of important animal and human pathogens, is closely associated with the cellular membrane compartments, especially the endoplasmic reticulum (ER). Coronavirus infection of cultured cells was previously shown to cause ER stress and induce the unfolded protein response (UPR), a process that aims to restore the ER homeostasis by global translation shutdown and increasing the ER folding capacity. However, under prolonged ER stress, UPR can also induce apoptotic cell death. Accumulating evidence from recent studies has shown that induction of ER stress and UPR may constitute a major aspect of coronavirus-host interaction. Activation of the three branches of UPR modulates a wide variety of signaling pathways, such as mitogen-activated protein (MAP) kinase activation, autophagy, apoptosis, and innate immune response. ER stress and UPR activation may therefore contribute significantly to the viral replication and pathogenesis during coronavirus infection. In this review, we summarize the current knowledge on coronavirus-induced ER stress and UPR activation, with emphasis on their cross-talking to apoptotic signaling.
Keywords: ER stress; apoptosis; coronavirus; proinflammatory cytokines; signal transduction pathways; unfolded protein response.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

