AGROBEST: an efficient Agrobacterium-mediated transient expression method for versatile gene function analyses in Arabidopsis seedlings
- PMID: 24987449
- PMCID: PMC4076510
- DOI: 10.1186/1746-4811-10-19
AGROBEST: an efficient Agrobacterium-mediated transient expression method for versatile gene function analyses in Arabidopsis seedlings
Abstract
Background: Transient gene expression via Agrobacterium-mediated DNA transfer offers a simple and fast method to analyze transgene functions. Although Arabidopsis is the most-studied model plant with powerful genetic and genomic resources, achieving highly efficient and consistent transient expression for gene function analysis in Arabidopsis remains challenging.
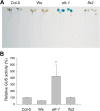
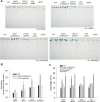
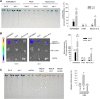
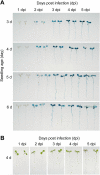
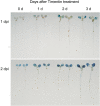
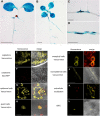
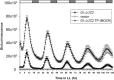
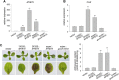
Results: We developed a highly efficient and robust Agrobacterium-mediated transient expression system, named AGROBEST (Agrobacterium-mediated enhanced seedling transformation), which achieves versatile analysis of diverse gene functions in intact Arabidopsis seedlings. Using β-glucuronidase (GUS) as a reporter for Agrobacterium-mediated transformation assay, we show that the use of a specific disarmed Agrobacterium strain with vir gene pre-induction resulted in homogenous GUS staining in cotyledons of young Arabidopsis seedlings. Optimization with AB salts in plant culture medium buffered with acidic pH 5.5 during Agrobacterium infection greatly enhanced the transient expression levels, which were significantly higher than with two existing methods. Importantly, the optimized method conferred 100% infected seedlings with highly increased transient expression in shoots and also transformation events in roots of ~70% infected seedlings in both the immune receptor mutant efr-1 and wild-type Col-0 seedlings. Finally, we demonstrated the versatile applicability of the method for examining transcription factor action and circadian reporter-gene regulation as well as protein subcellular localization and protein-protein interactions in physiological contexts.
Conclusions: AGROBEST is a simple, fast, reliable, and robust transient expression system enabling high transient expression and transformation efficiency in Arabidopsis seedlings. Demonstration of the proof-of-concept experiments elevates the transient expression technology to the level of functional studies in Arabidopsis seedlings in addition to previous applications in fluorescent protein localization and protein-protein interaction studies. In addition, AGROBEST offers a new way to dissect the molecular mechanisms involved in Agrobacterium-mediated DNA transfer.
Keywords: Agrobacterium; Arabidopsis; Gain-of-function; Gene expression; Innate immunity; Transient transformation.
Figures


References
-
- Dandekar AM, Fisk HJ. Plant transformation: Agrobacterium-mediated gene transfer. Methods Mol Biol. 2005;286:35–46. - PubMed
-
- Gelvin SB. Agricultural biotechnology: gene exchange by design. Nature. 2005;433:583–584. - PubMed
-
- Tzfira T, Citovsky V. Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol. 2006;17:147–154. - PubMed
-
- Gelvin SB. Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol. 2010;48:45–68. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

