Estimation of diffusion coefficients from voltammetric signals by support vector and gaussian process regression
- PMID: 24987463
- PMCID: PMC4074154
- DOI: 10.1186/1758-2946-6-30
Estimation of diffusion coefficients from voltammetric signals by support vector and gaussian process regression
Abstract
Background: Support vector regression (SVR) and Gaussian process regression (GPR) were used for the analysis of electroanalytical experimental data to estimate diffusion coefficients.
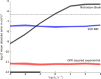
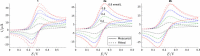
Results: For simulated cyclic voltammograms based on the EC, Eqr, and EqrC mechanisms these regression algorithms in combination with nonlinear kernel/covariance functions yielded diffusion coefficients with higher accuracy as compared to the standard approach of calculating diffusion coefficients relying on the Nicholson-Shain equation. The level of accuracy achieved by SVR and GPR is virtually independent of the rate constants governing the respective reaction steps. Further, the reduction of high-dimensional voltammetric signals by manual selection of typical voltammetric peak features decreased the performance of both regression algorithms compared to a reduction by downsampling or principal component analysis. After training on simulated data sets, diffusion coefficients were estimated by the regression algorithms for experimental data comprising voltammetric signals for three organometallic complexes.
Conclusions: Estimated diffusion coefficients closely matched the values determined by the parameter fitting method, but reduced the required computational time considerably for one of the reaction mechanisms. The automated processing of voltammograms according to the regression algorithms yields better results than the conventional analysis of peak-related data.
Keywords: Diffusion coefficient; Gaussian process regression; Principal component analysis; Reaction mechanism; Support vector regression; Voltammetry.
Figures









References
-
- Nicholson RS, Shain I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. 1964. pp. 706–723.
-
- Heinze J. Cyclovoltammetrie — die “Spektroskopie” des Elektrochemikers. Angew Chem. 1984;96:823–840. Cyclic Voltammetry — the “Spectroscopy” of the Electrochemist. Angew Chem Int Ed Engl 1984, 23:831–847.
-
- Economou A, Bolis SD, Efstathiou CE, Volikakis GJ. A “virtual” electroanalytical instrument for square wave voltammetry. Anal Chim Acta. 2002;467:179–188.
-
- Speiser B. In: Encyclopedia of Electrochemistry, Vol. 8 Organic Electrochemistry, Chap. 1. Bard AJ, Stratmann M, Schäfer HJ, editor. Weinheim: Wiley-VCH; 2004. Methods to investigate mechanisms of electroorganic reactions; pp. 1–23.
-
- Venton BJ, Wightman RM. Psychoanalytical electrochemistry: Dopamine and behavior. Anal Chem. 2003;75:414A–421A.
LinkOut - more resources
Full Text Sources
Other Literature Sources

