Copper(I)-catalyzed multicomponent reaction providing a new access to fully substituted thiophene derivatives
- PMID: 24988049
- PMCID: PMC4337423
- DOI: 10.1021/ol501404x
Copper(I)-catalyzed multicomponent reaction providing a new access to fully substituted thiophene derivatives
Abstract
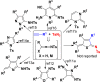
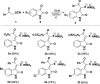
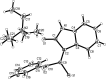
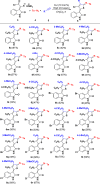
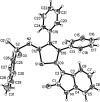
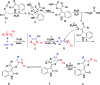
Readily available triethylammonium 1-(2-oxoindolin-3-ylidene)-2-aroylethanethiolates are efficiently converted into a variety of fully substituted thiophene derivatives by copper(I)-catalyzed denitrogenative reactions with terminal alkynes and N-sulfonyl azides. This new reaction simultaneously installs C-N, C-S, and C-C bonds, allowing direct formation of highly functionalized thiophenes with a wide diversity in substituents in a one-pot manner. A plausible mechanism for the domino process is proposed.
Figures


References
-
- Russell R. K.; Press J. B. In Comprehensive Heterocyclic Chemistry II; Katritzky A. R., Rees C. W., Scriven E. W. F., Padwa A., Eds.; Pergamon: New York, 1996; Vol. 2, pp 679–729.
- Goeb S.; De Nicola A.; Ziessel R. Synlett 2005, 1169–1177.
- Jesberger M.; Davis T. P.; Barner L. Synthesis 2003, 1929–1958.
- Koike K.; Jia Z.; Nikaido T.; Liu Y.; Zhao Y.; Guo D. Org. Lett. 1999, 1, 197–198.
-
- Pinkerton A. B.; Lee T. T.; Hoffman T. Z.; Wang Y.; Kahraman M.; Cook T. G.; Severance D.; Gahman T. C.; Noble S. A.; Shiau A. K.; Davis R. L. Bioorg. Med. Chem. Lett. 2007, 17, 3562. - PubMed
-
- Takimiya K.; Nakano M.; Kang M. J.; Miyazaki E.; Osaka I. Eur. J. Org. Chem. 2013, 217.
- Perepichka I. F.; Perepichka D. F.. Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics; Wiley-VCH: Weinheim, 2009.
- Krebs F. C.Polymer Photovoltaics: A Practical Approach; SPIE: Bellingham, 2008.
-
- Gueney S.; Becerik I.; Kadirgan F. Bull. Electrochem. 2004, 20, 157.
- Ng S. C.; Fu P.; Yu W.-L.; Chan H. S. O.; Tan K. L. Synth. Metal. 1997, 87, 119.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

