The Ran GTPase-activating protein (RanGAP1) is critically involved in smooth muscle cell differentiation, proliferation and migration following vascular injury: implications for neointima formation and restenosis
- PMID: 24988324
- PMCID: PMC4079658
- DOI: 10.1371/journal.pone.0101519
The Ran GTPase-activating protein (RanGAP1) is critically involved in smooth muscle cell differentiation, proliferation and migration following vascular injury: implications for neointima formation and restenosis
Abstract
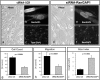
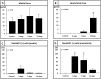
Differentiation and dedifferentiation, accompanied by proliferation play a pivotal role for the phenotypic development of vascular proliferative diseases (VPD), such as restenosis. Increasing evidence points to an essential role of regulated nucleoporin expression in the choice between differentiation and proliferation. However, whether components of the Ran GTPase cycle, which is of pivotal importance for both nucleocytoplasmic transport and for mitotic progression, are subject to similar regulation in VPD is currently unknown. Here, we show that differentiation of human coronary artery smooth muscle cell (CASMC) to a contractile phenotype by stepwise serum depletion leads to significant reduction of RanGAP1 protein levels. The inverse event, dedifferentiation of cells, was assessed in the rat carotid artery balloon injury model, a well-accepted model for neointima formation and restenosis. As revealed by temporospatial analysis of RanGAP1 expression, neointima formation in rat carotid arteries was associated with a significant upregulation of RanGAP1 expression at 3 and 7 days after balloon injury. Of note, neointimal cells located at the luminal surface revealed persistent RanGAP1 expression, as opposed to cells in deeper layers of the neointima where RanGAP1 expression was less or not detectable at all. To gain first evidence for a direct influence of RanGAP1 levels on differentiation, we reduced RanGAP1 in human coronary artery smooth muscle cells by siRNA. Indeed, downregulation of the essential RanGAP1 protein by 50% induced a differentiated, spindle-like smooth muscle cell phenotype, accompanied by an upregulation of the differentiation marker desmin. Reduction of RanGAP1 levels also resulted in a reduction of mitogen induced cellular migration and proliferation as well as a significant upregulation of the cyclin-dependent kinase inhibitor p27KIP1, without evidence for cellular necrosis. These findings suggest that RanGAP1 plays a critical role in smooth muscle cell differentiation, migration and proliferation in vitro and in vivo. Appropriate modulation of RanGAP1 expression may thus be a strategy to modulate VPD development such as restenosis.
Conflict of interest statement
Figures


Similar articles
-
Suv39h1 downregulation inhibits neointimal hyperplasia after vascular injury.Atherosclerosis. 2019 Sep;288:76-84. doi: 10.1016/j.atherosclerosis.2019.06.909. Epub 2019 Jun 20. Atherosclerosis. 2019. PMID: 31330382
-
Regulator of G-Protein Signaling 5 Prevents Smooth Muscle Cell Proliferation and Attenuates Neointima Formation.Arterioscler Thromb Vasc Biol. 2016 Feb;36(2):317-27. doi: 10.1161/ATVBAHA.115.305974. Epub 2015 Dec 10. Arterioscler Thromb Vasc Biol. 2016. PMID: 26663397
-
Local cyclin-dependent kinase inhibition by flavopiridol inhibits coronary artery smooth muscle cell proliferation and migration: Implications for the applicability on drug-eluting stents to prevent neointima formation following vascular injury.FASEB J. 2004 Aug;18(11):1285-7. doi: 10.1096/fj.04-1646fje. Epub 2004 Jun 4. FASEB J. 2004. PMID: 15180955
-
Biological responses in stented arteries.Cardiovasc Res. 2013 Jul 15;99(2):353-63. doi: 10.1093/cvr/cvt115. Epub 2013 May 10. Cardiovasc Res. 2013. PMID: 23667187 Review.
-
The role of extracellular vesicles in neointima formation post vascular injury.Cell Signal. 2020 Dec;76:109783. doi: 10.1016/j.cellsig.2020.109783. Epub 2020 Sep 18. Cell Signal. 2020. PMID: 32956789 Review.
Cited by
-
Hepatitis B virus core protein stabilizes RANGAP1 to upregulate KDM2A and facilitate hepatocarcinogenesis.Cell Oncol (Dordr). 2024 Apr;47(2):639-655. doi: 10.1007/s13402-023-00889-4. Epub 2023 Oct 17. Cell Oncol (Dordr). 2024. PMID: 37845585
-
Direct active Fyn-paxillin interaction regulates vascular smooth muscle cell migration.J Smooth Muscle Res. 2023;59:58-66. doi: 10.1540/jsmr.59.58. J Smooth Muscle Res. 2023. PMID: 37438114 Free PMC article. Review.
-
Association of Polymorphisms in miRNA Processing Genes With Type 2 Diabetes Mellitus and Its Vascular Complications in a Southern Chinese Population.Front Endocrinol (Lausanne). 2019 Jul 12;10:461. doi: 10.3389/fendo.2019.00461. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31354628 Free PMC article.
-
Nucleoporins in cardiovascular disease.J Mol Cell Cardiol. 2020 Apr;141:43-52. doi: 10.1016/j.yjmcc.2020.02.010. Epub 2020 Mar 21. J Mol Cell Cardiol. 2020. PMID: 32209327 Free PMC article. Review.
-
The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes.Nat Commun. 2016 May 10;7:11482. doi: 10.1038/ncomms11482. Nat Commun. 2016. PMID: 27160050 Free PMC article.
References
-
- Ross R (1999) Atherosclerosis is an inflammatory disease. Am Heart J 138: 419–20. - PubMed
-
- Dzau VJ, Braun-Dullaeus RC, Sedding DG (2002) Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 8: 1249–56. - PubMed
-
- Libby P, Aikawa M (2002) Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med 8: 1257–62. - PubMed
-
- Libby P, O‘Brien KV (1984) The role of protein breakdown in growth, quiescence and starvation of vascular smooth muscle cells. J Cell Physiol 118: 317–323. - PubMed
-
- Ross R, Glomset JA (1973) Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 180: 1332–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

