Catch-and-hold activation of muscle acetylcholine receptors having transmitter binding site mutations
- PMID: 24988344
- PMCID: PMC4119287
- DOI: 10.1016/j.bpj.2014.04.057
Catch-and-hold activation of muscle acetylcholine receptors having transmitter binding site mutations
Abstract
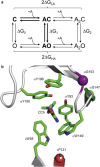
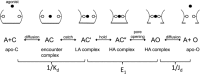
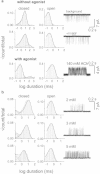
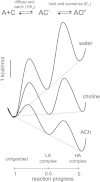
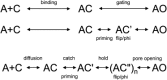
Agonists turn on receptors because their target sites have a higher affinity in the active versus resting conformation of the protein. We used single-channel electrophysiology to measure the lower-affinity (LA) and higher-affinity (HA) equilibrium dissociation constants for acetylcholine in adult-type muscle mouse nicotinic receptors (AChRs) having mutations of agonist binding site amino acids. For a series of agonists and for all mutations of αY93, αG147, αW149, αY190, αY198, εW55, and δW57, the change in LA binding energy was approximately half that in HA binding energy. The results were analyzed as a linear free energy relationship between LA and HA agonist binding, the slope of which (κ) gives the fraction of the overall binding chemical potential where the LA complex is established. The linear correlation between LA and HA binding energies suggests that the overall binding process is by an integrated mechanism (catch-and-hold). For the agonist and the above mutations, κ ∼ 0.5, but side-chain substitutions of two residues had a slope that was significantly higher (0.90; αG153) or lower (0.25; εP121). The results suggest that backbone rearrangements in loop B, loop C, and the non-α surface participate in both LA binding and the LA ↔ HA affinity switch. It appears that all of the intermediate steps in AChR activation comprise a single, energetically coupled process.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Don't flip out: AChRs are primed to catch and hold your attention.Biophys J. 2014 Jul 1;107(1):8-9. doi: 10.1016/j.bpj.2014.05.021. Biophys J. 2014. PMID: 24988335 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

