Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells
- PMID: 24989059
- PMCID: PMC4094603
- DOI: 10.1186/1471-2180-14-177
Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells
Abstract
Background: Clostridium difficile is the main cause of hospital-acquired diarrhea and colitis known as C. difficile-associated disease (CDAD).With increased severity and failure of treatment in CDAD, new approaches for prevention and treatment, such as the use of probiotics, are needed. Since the pathogenesis of CDAD involves an inflammatory response with a massive influx of neutrophils recruited by interleukin (IL)-8, this study aimed to investigate the probiotic effects of Lactobacillus spp. on the suppression of IL-8 production in response to C. difficile infection.
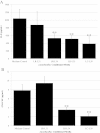
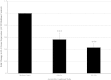
Results: We screened Lactobacillus conditioned media from 34 infant fecal isolates for the ability to suppress C. difficile-induced IL-8 production from HT-29 cells. Factors produced by two vancomycin-resistant lactobacilli, L. rhamnosus L34 (LR-L34) and L.casei L39 (LC-L39), suppressed the secretion and transcription of IL-8 without inhibiting C. difficile viability or toxin production. Conditioned media from LR-L34 suppressed the activation of phospho-NF-κB with no effect on phospho-c-Jun. However, LC-L39 conditioned media suppressed the activation of both phospho-NF-κB and phospho-c-Jun. Conditioned media from LR-L34 and LC-L39 also decreased the production of C. difficile-induced GM-CSF in HT-29 cells. Immunomodulatory factors present in the conditioned media of both LR-L34 and LC-L39 are heat-stable up to 100°C and > 100 kDa in size.
Conclusions: Our results suggest that L. rhamnosus L34 and L. casei L39 each produce factors capable of modulating inflammation stimulated by C. difficile. These vancomycin-resistant Lactobacillus strains are potential probiotics for treating or preventing CDAD.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

