The fidelity of the ligation step determines how ends are resolved during nonhomologous end joining
- PMID: 24989324
- PMCID: PMC4107315
- DOI: 10.1038/ncomms5286
The fidelity of the ligation step determines how ends are resolved during nonhomologous end joining
Abstract
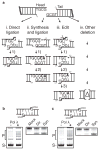
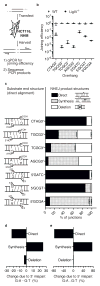
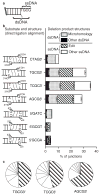
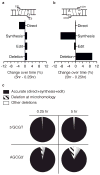
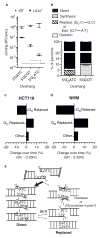
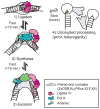
Nonhomologous end joining (NHEJ) can effectively resolve chromosome breaks despite diverse end structures; however, it is unclear how the steps employed for resolution are determined. We sought to address this question by analysing cellular NHEJ of ends with systematically mispaired and damaged termini. We show NHEJ is uniquely proficient at bypassing subtle terminal mispairs and radiomimetic damage by direct ligation. Nevertheless, bypass ability varies widely, with increases in mispair severity gradually reducing bypass products from 85 to 6%. End-processing by nucleases and polymerases is increased to compensate, although paths with the fewest number of steps to generate a substrate suitable for ligation are favoured. Thus, both the frequency and nature of end processing are tailored to meet the needs of the ligation step. We propose a model where the ligase organizes all steps during NHEJ within the stable paired-end complex to limit end processing and associated errors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- O’Driscoll M, Jeggo PA. The role of double-strand break repair —insights from human genetics. Nat Rev Genet. 2006;7:45–54. - PubMed
-
- Dobbs TA, Palmer P, Maniou Z, Lomax ME, O’Neill P. Interplay of two major repair pathways in the processing of complex double-strand DNA breaks. DNA Repair (Amst ) 2008;7:1372–1383. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

