Deformable segmentation of 3D MR prostate images via distributed discriminative dictionary and ensemble learning
- PMID: 24989402
- PMCID: PMC4105964
- DOI: 10.1118/1.4884224
Deformable segmentation of 3D MR prostate images via distributed discriminative dictionary and ensemble learning
Abstract
Purpose: Automatic prostate segmentation from MR images is an important task in various clinical applications such as prostate cancer staging and MR-guided radiotherapy planning. However, the large appearance and shape variations of the prostate in MR images make the segmentation problem difficult to solve. Traditional Active Shape/Appearance Model (ASM/AAM) has limited accuracy on this problem, since its basic assumption, i.e., both shape and appearance of the targeted organ follow Gaussian distributions, is invalid in prostate MR images. To this end, the authors propose a sparse dictionary learning method to model the image appearance in a nonparametric fashion and further integrate the appearance model into a deformable segmentation framework for prostate MR segmentation.
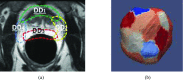
Methods: To drive the deformable model for prostate segmentation, the authors propose nonparametric appearance and shape models. The nonparametric appearance model is based on a novel dictionary learning method, namely distributed discriminative dictionary (DDD) learning, which is able to capture fine distinctions in image appearance. To increase the differential power of traditional dictionary-based classification methods, the authors' DDD learning approach takes three strategies. First, two dictionaries for prostate and nonprostate tissues are built, respectively, using the discriminative features obtained from minimum redundancy maximum relevance feature selection. Second, linear discriminant analysis is employed as a linear classifier to boost the optimal separation between prostate and nonprostate tissues, based on the representation residuals from sparse representation. Third, to enhance the robustness of the authors' classification method, multiple local dictionaries are learned for local regions along the prostate boundary (each with small appearance variations), instead of learning one global classifier for the entire prostate. These discriminative dictionaries are located on different patches of the prostate surface and trained to adaptively capture the appearance in different prostate zones, thus achieving better local tissue differentiation. For each local region, multiple classifiers are trained based on the randomly selected samples and finally assembled by a specific fusion method. In addition to this nonparametric appearance model, a prostate shape model is learned from the shape statistics using a novel approach, sparse shape composition, which can model nonGaussian distributions of shape variation and regularize the 3D mesh deformation by constraining it within the observed shape subspace.
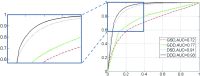
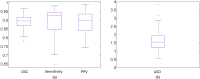
Results: The proposed method has been evaluated on two datasets consisting of T2-weighted MR prostate images. For the first (internal) dataset, the classification effectiveness of the authors' improved dictionary learning has been validated by comparing it with three other variants of traditional dictionary learning methods. The experimental results show that the authors' method yields a Dice Ratio of 89.1% compared to the manual segmentation, which is more accurate than the three state-of-the-art MR prostate segmentation methods under comparison. For the second dataset, the MICCAI 2012 challenge dataset, the authors' proposed method yields a Dice Ratio of 87.4%, which also achieves better segmentation accuracy than other methods under comparison.
Conclusions: A new magnetic resonance image prostate segmentation method is proposed based on the combination of deformable model and dictionary learning methods, which achieves more accurate segmentation performance on prostate T2 MR images.
Figures









Similar articles
-
MR PROSTATE SEGMENTATION VIA DISTRIBUTED DISCRIMINATIVE DICTIONARY (DDD) LEARNING.Proc IEEE Int Symp Biomed Imaging. 2013;2013:868-871. doi: 10.1109/ISBI.2013.6556613. Proc IEEE Int Symp Biomed Imaging. 2013. PMID: 25035792 Free PMC article.
-
Automatic prostate MR image segmentation with sparse label propagation and domain-specific manifold regularization.Inf Process Med Imaging. 2013;23:511-23. doi: 10.1007/978-3-642-38868-2_43. Inf Process Med Imaging. 2013. PMID: 24683995 Free PMC article.
-
Deformable MR Prostate Segmentation via Deep Feature Learning and Sparse Patch Matching.IEEE Trans Med Imaging. 2016 Apr;35(4):1077-89. doi: 10.1109/TMI.2015.2508280. Epub 2015 Dec 11. IEEE Trans Med Imaging. 2016. PMID: 26685226 Free PMC article.
-
Deep Learning Prostate MRI Segmentation Accuracy and Robustness: A Systematic Review.Radiol Artif Intell. 2024 Jul;6(4):e230138. doi: 10.1148/ryai.230138. Radiol Artif Intell. 2024. PMID: 38568094 Free PMC article.
-
Investigation and benchmarking of U-Nets on prostate segmentation tasks.Comput Med Imaging Graph. 2023 Jul;107:102241. doi: 10.1016/j.compmedimag.2023.102241. Epub 2023 May 12. Comput Med Imaging Graph. 2023. PMID: 37201475 Review.
Cited by
-
Superpixel-Based Segmentation for 3D Prostate MR Images.IEEE Trans Med Imaging. 2016 Mar;35(3):791-801. doi: 10.1109/TMI.2015.2496296. Epub 2015 Oct 30. IEEE Trans Med Imaging. 2016. PMID: 26540678 Free PMC article.
-
A supervoxel-based segmentation method for prostate MR images.Proc SPIE Int Soc Opt Eng. 2015 Mar 20;9413:941318. doi: 10.1117/12.2082255. Proc SPIE Int Soc Opt Eng. 2015. PMID: 26848206 Free PMC article.
-
Deeply supervised 3D fully convolutional networks with group dilated convolution for automatic MRI prostate segmentation.Med Phys. 2019 Apr;46(4):1707-1718. doi: 10.1002/mp.13416. Epub 2019 Feb 19. Med Phys. 2019. PMID: 30702759 Free PMC article.
-
Liver segmentation from CT images using a sparse priori statistical shape model (SP-SSM).PLoS One. 2017 Oct 5;12(10):e0185249. doi: 10.1371/journal.pone.0185249. eCollection 2017. PLoS One. 2017. PMID: 28981530 Free PMC article.
-
Automated Segmentation of Tissues Using CT and MRI: A Systematic Review.Acad Radiol. 2019 Dec;26(12):1695-1706. doi: 10.1016/j.acra.2019.07.006. Epub 2019 Aug 10. Acad Radiol. 2019. PMID: 31405724 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

