A protein-based pentavalent inhibitor of the cholera toxin B-subunit
- PMID: 24989497
- PMCID: PMC4499251
- DOI: 10.1002/anie.201404397
A protein-based pentavalent inhibitor of the cholera toxin B-subunit
Abstract
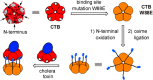
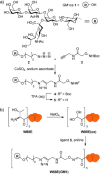
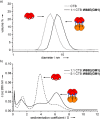
Protein toxins produced by bacteria are the cause of many life-threatening diarrheal diseases. Many of these toxins, including cholera toxin (CT), enter the cell by first binding to glycolipids in the cell membrane. Inhibiting these multivalent protein/carbohydrate interactions would prevent the toxin from entering cells and causing diarrhea. Here we demonstrate that the site-specific modification of a protein scaffold, which is perfectly matched in both size and valency to the target toxin, provides a convenient route to an effective multivalent inhibitor. The resulting pentavalent neoglycoprotein displays an inhibition potency (IC50) of 104 pM for the CT B-subunit (CTB), which is the most potent pentavalent inhibitor for this target reported thus far. Complexation of the inhibitor and CTB resulted in a protein heterodimer. This inhibition strategy can potentially be applied to many multivalent receptors and also opens up new possibilities for protein assembly strategies.
Keywords: carbohydrates; glycoproteins; multivalency; protein modifications; protein structures.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures

References
-
- Vanden Broeck D, Horvath C, De Wolf MJS. Int. J. Biochem. Cell Biol. 2007;39:1771–1775. - PubMed
-
- Branson TR, Turnbull WB. Chem. Soc. Rev. 2013;42:4613–4622. - PubMed
-
- Bernardi A, Jimenez-Barbero J, Casnati A, De Castro C, Darbre T, Fieschi F, Finne J, Funken H, Jaeger K-E, Lahmann M, Lindhorst TK, Marradi M, Messner P, Molinaro A, Murphy PV, Nativi C, Oscarson S, Penades S, Peri F, Pieters RJ, Renaudet O, Reymond J-L, Richichi B, Rojo J, Sansone F, Schaffer C, Turnbull WB, Velasco-Torrijos T, Vidal S, Vincent S, Wennekes T, Zuilhof H, Imberty A. Chem. Soc. Rev. 2013;42:4709–4727. - PMC - PubMed
-
- Pieters RJ. Org. Biomol. Chem. 2009;7:2013–2025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

