Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study
- PMID: 24989666
- PMCID: PMC4155185
- DOI: 10.1007/s00415-014-7412-x
Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study
Abstract
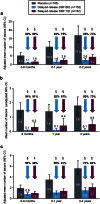
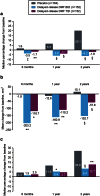
In the Phase 3 DEFINE study, delayed-release dimethyl fumarate (DMF) 240 mg twice (BID) and three times daily (TID) significantly reduced the mean number of new or enlarging T2-hyperintense lesions and gadolinium-enhancing (Gd+) lesion activity at 2 years in patients (MRI cohort; n = 540) with relapsing-remitting MS. The analyses described here expand on these results by considering additional MRI measures (number of T1-hypointense lesions; volume of T2-hyperintense, Gd+, and T1-hypointense lesions; brain atrophy), delineating the time course of the effects, and examining the generality of the effects across a diverse patient population. Reductions in lesion counts with delayed-release DMF BID and TID, respectively, vs. placebo were apparent by the first MRI assessment at 6 months [T2-hyperintense: 80 and 69 % reduction (both P < 0.0001); Gd+, 94 and 81 % reduction (both P < 0.0001); T1-hypointense: 58 % (P < 0.0001) and 48 % (P = 0.0005) reduction] and maintained at 1 and 2 years. Reductions in lesion volume were statistically significant beginning at 6 months for T2-hyperintense [P = 0.0002 (BID) and P = 0.0035 (TID)] and Gd+ lesions [P = 0.0059 (BID) and P = 0.0176 (TID)] and beginning at 1 year [P = 0.0126 (BID)] to 2 years [P = 0.0063 (TID)] for T1-hypointense lesions. Relative reductions in brain atrophy from baseline to 2 years (21 % reduction; P = 0.0449) and 6 months to 2 years (30 % reduction; P = 0.0214) were statistically significant for delayed-release DMF BID. The effect of delayed-release DMF on mean number of new or enlarging T2-hyperintense lesions and Gd+ lesion activity was consistent across pre-specified patient subpopulations. Collectively, these results suggest that delayed-release DMF favorably affects multiple aspects of MS pathophysiology.
Figures

References
-
- Kappos L, Moeri D, Radue EW, Schoetzau A, Schweikert K, Barkhof F, Miller D, Guttmann CR, Weiner HL, Gasperini C, Filippi M. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet. 1999;353(9157):964–969. doi: 10.1016/S0140-6736(98)03053-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

