Stereodivergent organocatalytic intramolecular Michael addition/lactonization for the asymmetric synthesis of substituted dihydrobenzofurans and tetrahydrofurans
- PMID: 24989672
- PMCID: PMC4517160
- DOI: 10.1002/chem.201402684
Stereodivergent organocatalytic intramolecular Michael addition/lactonization for the asymmetric synthesis of substituted dihydrobenzofurans and tetrahydrofurans
Abstract
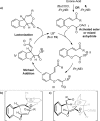
A stereodivergent asymmetric Lewis base catalyzed Michael addition/lactonization of enone acids into substituted dihydrobenzofuran and tetrahydrofuran derivatives is reported. Commercially available (S)-(-)-tetramisole hydrochloride gives products with high syn diastereoselectivity in excellent enantioselectivity (up to 99:1 d.r.syn/anti , 99 % eesyn ), whereas using a cinchona alkaloid derived catalyst gives the corresponding anti-diastereoisomers as the major product (up to 10:90 d.r.syn/anti , 99 % eeanti ).
Keywords: Michael addition; asymmetric catalysis; cinchona alkaloid; isothiourea; organocatalysis; oxygen heterocycles; stereodivergent.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


Similar articles
-
Enantioselective synthesis of 2,3-disubstituted trans-2,3-dihydrobenzofurans using a Brønsted base/thiourea bifunctional catalyst.Org Biomol Chem. 2016 Jul 26;14(30):7268-74. doi: 10.1039/c6ob01326k. Org Biomol Chem. 2016. PMID: 27387095
-
Stereocontrolled organocatalytic strategy for the synthesis of optically active 2,3-disubstituted cis-2,3-dihydrobenzofurans.Chem Asian J. 2013 Mar;8(3):648-52. doi: 10.1002/asia.201201072. Epub 2012 Dec 13. Chem Asian J. 2013. PMID: 23239582
-
Organocatalytic functionalization of carboxylic acids: isothiourea-catalyzed asymmetric intra- and intermolecular Michael addition--lactonizations.J Am Chem Soc. 2011 Mar 2;133(8):2714-20. doi: 10.1021/ja109975c. Epub 2011 Feb 8. J Am Chem Soc. 2011. PMID: 21302961
-
Reactions of o-quinone methides with pyridinium methylides: a diastereoselective synthesis of 1,2-dihydronaphtho[2,1-b]furans and 2,3-dihydrobenzofurans.J Org Chem. 2013 Jun 7;78(11):5505-20. doi: 10.1021/jo400621r. Epub 2013 May 22. J Org Chem. 2013. PMID: 23668240
-
[Development of Organocatalytic Reactions for the Synthesis of Natural Products and Pharmaceuticals].Yakugaku Zasshi. 2024;144(8):791-798. doi: 10.1248/yakushi.24-00095. Yakugaku Zasshi. 2024. PMID: 39085055 Review. Japanese.
Cited by
-
Diastereodivergent cis- and trans-fused [4 + 2] annulations of cyclic 1,3-dienes and 1-azadienes via ligand-controlled palladium catalysis.Chem Sci. 2023 Jan 17;14(7):1896-1901. doi: 10.1039/d2sc06813c. eCollection 2023 Feb 15. Chem Sci. 2023. PMID: 36819872 Free PMC article.
-
Enantioselective synthesis of cyclopenta[b]benzofurans via an organocatalytic intramolecular double cyclization.Chem Sci. 2017 Dec 1;8(12):8086-8093. doi: 10.1039/c7sc03006a. Epub 2017 Oct 2. Chem Sci. 2017. PMID: 29568457 Free PMC article.
-
6-exo-trig Michael addition-lactonizations for catalytic enantioselective chromenone synthesis.Chem Commun (Camb). 2017 Feb 23;53(17):2555-2558. doi: 10.1039/c6cc10178j. Chem Commun (Camb). 2017. PMID: 28133660 Free PMC article.
-
Generation and Reactivity of C(1)-Ammonium Enolates by Using Isothiourea Catalysis.Chemistry. 2021 Jan 21;27(5):1533-1555. doi: 10.1002/chem.202002059. Epub 2020 Nov 10. Chemistry. 2021. PMID: 32557875 Free PMC article. Review.
-
Quantum Reality in the Selective Reduction of a Benzofuran System.Molecules. 2019 May 30;24(11):2061. doi: 10.3390/molecules24112061. Molecules. 2019. PMID: 31151186 Free PMC article.
References
-
- Lorente A, Lamariano-Merketegi J, Albericio F, Alvarez M. Chem. Rev. 2013;113:4567–4610. - PubMed
-
- Goel A, Kumar A, Raghuvanshi A. Chem. Rev. 2012;112:1614–1640. - PubMed
-
- Gallimore AR. Nat. Prod. Rep. 2009;26:266–280. - PubMed
-
- Saleem M, Kim HJ, Ali MS, Lee YS. Nat. Prod. Rep. 2005;22:696–716. - PubMed
-
- Kang EJ, Lee E. Chem. Rev. 2005;105:4348–4378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

