Ena/VASP regulates mDia2-initiated filopodial length, dynamics, and function
- PMID: 24989797
- PMCID: PMC4148250
- DOI: 10.1091/mbc.E14-02-0712
Ena/VASP regulates mDia2-initiated filopodial length, dynamics, and function
Abstract
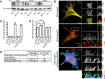
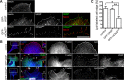
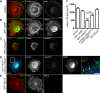
Filopodia are long plasma membrane extensions involved in the formation of adhesive, contractile, and protrusive actin-based structures in spreading and migrating cells. Whether filopodia formed by different molecular mechanisms equally support these cellular functions is unresolved. We used Enabled/vasodilator-stimulated phosphoprotein (Ena/VASP)-deficient MV(D7) fibroblasts, which are also devoid of endogenous mDia2, as a model system to investigate how these different actin regulatory proteins affect filopodia morphology and dynamics independently of one another. Filopodia initiated by either Ena/VASP or mDia2 contained similar molecular inventory but differed significantly in parameters such as number, length, F-actin organization, lifetime, and protrusive persistence. Moreover, in the absence of Ena/VASP, filopodia generated by mDia2 did not support initiation of integrin-dependent signaling cascades required for adhesion and subsequent lamellipodial extension, thereby causing a defect in early cell spreading. Coexpression of VASP with constitutively active mDia2(M/A) rescued these early adhesion defects. We conclude that Ena/VASP and mDia2 support the formation of filopodia with significantly distinct properties and that Ena/VASP regulates mDia2-initiated filopodial morphology, dynamics, and function.
© 2014 Barzik et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

