Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning
- PMID: 24989835
- PMCID: PMC5096299
- DOI: 10.1002/stem.1784
Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning
Erratum in
- Stem Cells. 2014 Dec;32(12):3287
Abstract
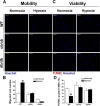
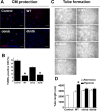
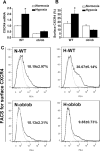
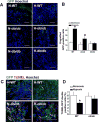
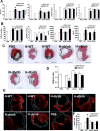
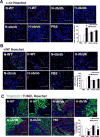
Hypoxia preconditioning enhances the therapeutic effect of mesenchymal stem cells (MSCs). However, the mechanism underlying hypoxia-induced augmentation of the protective effect of MSCs on myocardial infarction (MI) is poorly understood. We show that hypoxia-enhanced survival, mobility, and protection of cocultured cardiomyocytes were paralleled by increased expression of leptin and cell surface receptor CXCR4. The enhanced activities were abolished by either knockdown of leptin with a selective shRNA or by genetic deficiency of leptin or its receptor in MSCs derived, respectively, from ob/ob or db/db mice. To characterize the role of leptin in the regulation of MSC functions by hypoxia and its possible contribution to enhanced therapeutic efficacy, cell therapy using MSCs derived from wild-type, ob/ob, or db/db mice was implemented in mouse models of acute MI. Augmented protection by hypoxia pretreatment was only seen with MSCs from wild-type mice. Parameters that were differentially affected by hypoxia pretreatment included MSC engraftment, c-Kit(+) cell recruitment to the infarct, vascular density, infarct size, and long-term contractile function. These data show that leptin signaling is an early and essential step for the enhanced survival, chemotaxis, and therapeutic properties of MSCs conferred by preculture under hypoxia. Leptin may play a physiological role in priming MSCs resident in the bone marrow endosteum for optimal response to systemic signaling molecules and subsequent tissue repair.
Keywords: CXCR4; Hypoxic preconditioning; Leptin; Mesenchymal stem cells; Myocardial infarction.
© 2014 AlphaMed Press.
Conflict of interest statement
of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
Figures

Similar articles
-
Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury.Cell Biochem Funct. 2017 Mar;35(2):113-123. doi: 10.1002/cbf.3254. Epub 2017 Feb 23. Cell Biochem Funct. 2017. PMID: 28233339 Free PMC article.
-
LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning.Stem Cell Res Ther. 2018 Oct 25;9(1):280. doi: 10.1186/s13287-018-1031-x. Stem Cell Res Ther. 2018. PMID: 30359325 Free PMC article.
-
Follistatin-like 1 protects mesenchymal stem cells from hypoxic damage and enhances their therapeutic efficacy in a mouse myocardial infarction model.Stem Cell Res Ther. 2019 Jan 11;10(1):17. doi: 10.1186/s13287-018-1111-y. Stem Cell Res Ther. 2019. PMID: 30635025 Free PMC article.
-
Preconditioning influences mesenchymal stem cell properties in vitro and in vivo.J Cell Mol Med. 2018 Mar;22(3):1428-1442. doi: 10.1111/jcmm.13492. Epub 2018 Feb 1. J Cell Mol Med. 2018. PMID: 29392844 Free PMC article. Review.
-
Advances in application of hypoxia-preconditioned mesenchymal stem cell-derived exosomes.Front Cell Dev Biol. 2024 Aug 21;12:1446050. doi: 10.3389/fcell.2024.1446050. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39239560 Free PMC article. Review.
Cited by
-
Hypoxic Preconditioning Enhances Survival and Proangiogenic Capacity of Human First Trimester Chorionic Villus-Derived Mesenchymal Stem Cells for Fetal Tissue Engineering.Stem Cells Int. 2019 Nov 12;2019:9695239. doi: 10.1155/2019/9695239. eCollection 2019. Stem Cells Int. 2019. PMID: 31781252 Free PMC article.
-
Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction.Theranostics. 2019 Oct 11;9(24):7403-7416. doi: 10.7150/thno.32637. eCollection 2019. Theranostics. 2019. PMID: 31695776 Free PMC article.
-
The adipokine leptin modulates adventitial pericyte functions by autocrine and paracrine signalling.Sci Rep. 2017 Jul 14;7(1):5443. doi: 10.1038/s41598-017-05868-y. Sci Rep. 2017. PMID: 28710369 Free PMC article.
-
Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis.Stem Cell Res Ther. 2018 Mar 9;9(1):59. doi: 10.1186/s13287-018-0814-4. Stem Cell Res Ther. 2018. PMID: 29523186 Free PMC article. Review.
-
Stromal cells and stem cells in clinical bone regeneration.Nat Rev Endocrinol. 2015 Mar;11(3):140-50. doi: 10.1038/nrendo.2014.234. Epub 2015 Jan 6. Nat Rev Endocrinol. 2015. PMID: 25560703 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

