L-selectin deficiency decreases aortic B1a and Breg subsets and promotes atherosclerosis
- PMID: 24989887
- PMCID: PMC4344943
- DOI: 10.1160/TH13-10-0865
L-selectin deficiency decreases aortic B1a and Breg subsets and promotes atherosclerosis
Abstract
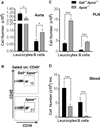
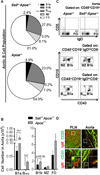
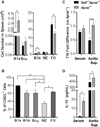
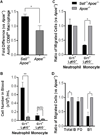
There is a significant recruitment of leucocytes into aortas during atherogenesis. L-selectin regulates leucocyte migration into secondary lymphoid and peripheral tissues and was proposed to play a role in leucocyte homing into aortas. Here, we determine the role of L-selectin in atherosclerosis. L-selectin-deficient Apoe-/- (Sell-/-Apoe-/-) mice had a 74% increase in plaque burden compared to Apoe-/- mice fed a chow diet for 50 weeks. Elevated atherosclerosis was accompanied by increased aortic leucocyte content, but a 50% reduction in aortic B cells despite elevated B cell counts in the blood. Follicular B cells represented 65%, whereas B1a and regulatory B cells (Breg) comprised 5% of aortic B cells. B1a and Breg cell subsets were reduced in Sell-/-Apoe-/- aortas with accompanied two-fold decrease in aortic T15 antibody and 1.2-fold decrease of interleukin-10 (IL-10) levels. L-selectin was required for B1 cell homing to the atherosclerotic aorta, as demonstrated by a 1.5-fold decrease in the migration of Sell-/-Apoe-/- vs Apoe-/- cells. Notably, we found a 1.6-fold increase in CD68hi macrophages in Sell-/-Apoe-/- compared to Apoe-/- aortas, despite comparable blood monocyte numbers and L-selectin-dependent aortic homing. L-selectin had no effect on neutrophil migration into aorta, but led to elevated blood neutrophil numbers, suggesting a potential involvement of neutrophils in atherogenesis of Sell-/-Apoe-/- mice. Thus, L-selectin deficiency increases peripheral blood neutrophil and lymphocyte numbers, decreases aortic B1a and Breg populations, T15 antibody and IL-10 levels, and increases aortic macrophage content of Sell-/-Apoe-/- mice. Altogether, these data provide evidence for an overall atheroprotective role of L-selectin.
Keywords: Atherosclerosis; B cell subsets; L-selectin.
Figures

Similar articles
-
Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A+ T Cells to the Aorta.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1496-506. doi: 10.1161/ATVBAHA.116.307892. Epub 2016 Jun 30. Arterioscler Thromb Vasc Biol. 2016. PMID: 27365405 Free PMC article.
-
P-Selectin Expressed by a Human SELP Transgene Is Atherogenic in Apolipoprotein E-Deficient Mice.Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1114-21. doi: 10.1161/ATVBAHA.116.307437. Epub 2016 Apr 21. Arterioscler Thromb Vasc Biol. 2016. PMID: 27102967 Free PMC article.
-
The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment.Circ Res. 2012 Mar 2;110(5):675-87. doi: 10.1161/CIRCRESAHA.111.261784. Epub 2012 Feb 2. Circ Res. 2012. PMID: 22302786 Free PMC article.
-
Deficiency in lymphotoxin β receptor protects from atherosclerosis in apoE-deficient mice.Circ Res. 2015 Apr 10;116(8):e57-68. doi: 10.1161/CIRCRESAHA.116.305723. Epub 2015 Mar 4. Circ Res. 2015. PMID: 25740843
-
Lymphocyte migration into atherosclerotic plaque.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):40-9. doi: 10.1161/ATVBAHA.114.303227. Epub 2014 Oct 9. Arterioscler Thromb Vasc Biol. 2015. PMID: 25301842 Free PMC article. Review.
Cited by
-
Deciphering mechanisms of cardiomyocytes and non-cardiomyocyte transformation in myocardial remodeling of permanent atrial fibrillation.J Adv Res. 2024 Jul;61:101-117. doi: 10.1016/j.jare.2023.09.012. Epub 2023 Sep 16. J Adv Res. 2024. PMID: 37722560 Free PMC article.
-
Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains.PLoS Genet. 2015 Dec 22;11(12):e1005711. doi: 10.1371/journal.pgen.1005711. eCollection 2015 Dec. PLoS Genet. 2015. PMID: 26694027 Free PMC article.
-
B cells and atherosclerosis.Am J Physiol Heart Circ Physiol. 2017 May 1;312(5):H1060-H1067. doi: 10.1152/ajpheart.00859.2016. Epub 2017 Mar 17. Am J Physiol Heart Circ Physiol. 2017. PMID: 28314764 Free PMC article. Review.
-
Where the Action Is-Leukocyte Recruitment in Atherosclerosis.Front Cardiovasc Med. 2022 Jan 11;8:813984. doi: 10.3389/fcvm.2021.813984. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35087886 Free PMC article. Review.
-
Regulatory B cells, interleukin-10, and atherosclerosis.Curr Opin Lipidol. 2015 Oct;26(5):470-1. doi: 10.1097/MOL.0000000000000220. Curr Opin Lipidol. 2015. PMID: 26339770 Free PMC article. No abstract available.
References
-
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

