The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy
- PMID: 24990037
- PMCID: PMC4453146
- DOI: 10.1259/bjr.20140134
The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy
Abstract
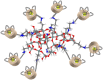
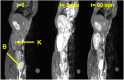
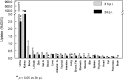
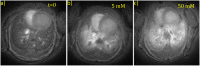
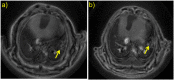
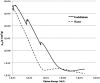
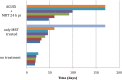
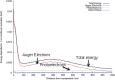
A new efficient type of gadolinium-based theranostic agent (AGuIX®) has recently been developed for MRI-guided radiotherapy (RT). These new particles consist of a polysiloxane network surrounded by a number of gadolinium chelates, usually 10. Owing to their small size (<5 nm), AGuIX typically exhibit biodistributions that are almost ideal for diagnostic and therapeutic purposes. For example, although a significant proportion of these particles accumulate in tumours, the remainder is rapidly eliminated by the renal route. In addition, in the absence of irradiation, the nanoparticles are well tolerated even at very high dose (10 times more than the dose used for mouse treatment). AGuIX particles have been proven to act as efficient radiosensitizers in a large variety of experimental in vitro scenarios, including different radioresistant cell lines, irradiation energies and radiation sources (sensitizing enhancement ratio ranging from 1.1 to 2.5). Pre-clinical studies have also demonstrated the impact of these particles on different heterotopic and orthotopic tumours, with both intratumoural or intravenous injection routes. A significant therapeutical effect has been observed in all contexts. Furthermore, MRI monitoring was proven to efficiently aid in determining a RT protocol and assessing tumour evolution following treatment. The usual theoretical models, based on energy attenuation and macroscopic dose enhancement, cannot account for all the results that have been obtained. Only theoretical models, which take into account the Auger electron cascades that occur between the different atoms constituting the particle and the related high radical concentrations in the vicinity of the particle, provide an explanation for the complex cell damage and death observed.
Figures





References
-
- Kwatra D, Venugopal A, Anant S. Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Transl Cancer Res 2013; 2: 330–42.
-
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004; 49: N309–15. - PubMed
-
- Butterworth KT, McMahon SJ, Taggart LE, Prise KM. Radiosensitization by gold nanoparticles: effective at megavoltage energies and potential role of oxidative stress. Transl Cancer Res 2013; 2: 279–89.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

