Histamine upregulates Nav1.8 expression in primary afferent neurons via H2 receptors: involvement in neuropathic pain
- PMID: 24990156
- PMCID: PMC6493056
- DOI: 10.1111/cns.12305
Histamine upregulates Nav1.8 expression in primary afferent neurons via H2 receptors: involvement in neuropathic pain
Abstract
Introduction: The upregulation of Nav1.8 in primary afferents plays a critical role in the development and persistence of neuropathic pain. The mechanisms underlying the upregulation are not fully understood.
Aims: The present study aims to investigate the regulatory effect of histamine on the expression of Nav1.8 in primary afferent neurons and its involvement in neuropathic pain.
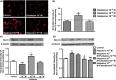
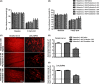
Results: Histamine at 10(-8) M increased the expression of Nav1.8 in cultured DRG neurons. This effect could be blocked by H2 receptor antagonist cimetidine or famotidine, but not by H1 receptor antagonist pyrilamine or dual H3 /H4 antagonist thioperamide. Peri-sciatic administration of histamine increased Nav1.8 expression in the sciatic nerve and L4/L5 DRG neurons in a dose-dependent manner, accompanied with remarkable mechanical allodynia and heat hyperalgesia in the ipsilateral hindpaw. Famotidine but not pyrilamine or thioperamide inhibited Nav1.8 upregulation and pain hypersensitivity. In addition, famotidine (40 mg/kg, i.p.) not only suppressed autotomy behavior in the rat neuroma model of neuropathic pain but also attenuated mechanical allodynia and thermal hyperalgesia following partial sciatic nerve ligation. Moreover, famotidine inhibited Nav1.8 upregulation in the neuroma and ligated sciatic nerve.
Conclusions: Our findings indicate that histamine increases Nav1.8 expression in primary afferent neurons via H2 receptor-mediated pathway and thereby contributes to neuropathic pain. H2 receptor antagonists may potentially be used as analgesics for patients with neuropathic pain.
Keywords: H2 antagonists; Histamine; Nav1.8; Neuropathic Pain; Primary afferents.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A critical time window for the analgesic effect of central histamine in the partial sciatic ligation model of neuropathic pain.J Neuroinflammation. 2016 Jun 24;13(1):163. doi: 10.1186/s12974-016-0637-0. J Neuroinflammation. 2016. PMID: 27342775 Free PMC article.
-
TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury.Pain. 2010 Nov;151(2):266-279. doi: 10.1016/j.pain.2010.06.005. Epub 2010 Jul 17. Pain. 2010. PMID: 20638792
-
Evaluation of different classes of histamine H1 and H2 receptor antagonist effects on neuropathic nociceptive behavior following tibial nerve transection in rats.Eur J Pharmacol. 2018 Sep 5;834:221-229. doi: 10.1016/j.ejphar.2018.07.011. Epub 2018 Jul 27. Eur J Pharmacol. 2018. PMID: 30009812
-
Famotidine Repurposing for Novel Corona Virus Disease of 2019: A Systematic Review.Drug Res (Stuttg). 2021 Jul;71(6):295-301. doi: 10.1055/a-1397-6763. Epub 2021 Mar 23. Drug Res (Stuttg). 2021. PMID: 33757133
-
Histamine receptors and COVID-19.Inflamm Res. 2021 Jan;70(1):67-75. doi: 10.1007/s00011-020-01422-1. Epub 2020 Nov 18. Inflamm Res. 2021. PMID: 33206207 Free PMC article.
Cited by
-
Transmission pathways and mediators as the basis for clinical pharmacology of pain.Expert Rev Clin Pharmacol. 2016 Oct;9(10):1363-1387. doi: 10.1080/17512433.2016.1204231. Epub 2016 Jul 4. Expert Rev Clin Pharmacol. 2016. PMID: 27322358 Free PMC article.
-
Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy?J Clin Med. 2022 May 6;11(9):2609. doi: 10.3390/jcm11092609. J Clin Med. 2022. PMID: 35566735 Free PMC article. Review.
-
[Pain in rheumatic diseases : What can biologics and JAK inhibitors offer?].Z Rheumatol. 2021 Apr;80(3):214-225. doi: 10.1007/s00393-020-00957-2. Epub 2021 Jan 14. Z Rheumatol. 2021. PMID: 33443608 Review. German.
-
The antinociception of oxytocin on colonic hypersensitivity in rats was mediated by inhibition of mast cell degranulation via Ca(2+)-NOS pathway.Sci Rep. 2016 Aug 19;6:31452. doi: 10.1038/srep31452. Sci Rep. 2016. PMID: 27538454 Free PMC article.
-
The problem of residual pain in the assessment of rheumatoid arthritis activity.Reumatologia. 2024;62(3):176-186. doi: 10.5114/reum/189779. Epub 2024 Jul 12. Reumatologia. 2024. PMID: 39055728 Free PMC article. Review.
References
-
- Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–819. - PubMed
-
- Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain‐related sensory abnormalities: A first step to a stratified treatment approach. Lancet Neurol 2012;11:999–1005. - PubMed
-
- Lai J, Gold MS, Kim CS, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin‐resistant sodium channel, NaV1.8. Pain 2002;95:143–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

