Human immature Langerhans cells restrict CXCR4-using HIV-1 transmission
- PMID: 24990163
- PMCID: PMC4227116
- DOI: 10.1186/1742-4690-11-52
Human immature Langerhans cells restrict CXCR4-using HIV-1 transmission
Abstract
Background: Sexual transmission is the main route of HIV-1 infection and the CCR5-using (R5) HIV-1 is predominantly transmitted, even though CXCR4-using (X4) HIV-1 is often abundant in chronic HIV-1 patients. The mechanisms underlying this tropism selection are unclear. Mucosal Langerhans cells (LCs) are the first immune cells to encounter HIV-1 and here we investigated the role of LCs in selection of R5 HIV-1 using an ex vivo epidermal and vaginal transmission models.
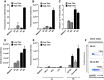
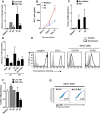
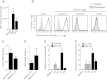
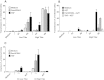
Results: Immature LCs were productively infected by X4 as well as R5 HIV-1. However, only R5 but not X4 viruses were selectively transmitted by immature LCs to T cells. Transmission of HIV-1 was depended on de novo production of HIV-1 in LCs, since it could be inhibited by CCR5 fusion inhibitors as well as reverse transcription inhibitors. Notably, the activation state of LCs affected the restriction in X4 HIV-1 transmission; immune activation by TNF facilitated transmission of X4 as well as R5 HIV-1.
Conclusions: These data suggest that LCs play a crucial role in R5 selection and that immature LCs effectively restrict X4 at the level of transmission.
Figures

Similar articles
-
R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms.Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8401-6. doi: 10.1073/pnas.1432450100. Epub 2003 Jun 18. Proc Natl Acad Sci U S A. 2003. PMID: 12815099 Free PMC article.
-
HIV-1 exposure and immune activation enhance sexual transmission of Hepatitis C virus by primary Langerhans cells.J Int AIDS Soc. 2019 Mar;22(3):e25268. doi: 10.1002/jia2.25268. J Int AIDS Soc. 2019. PMID: 30932366 Free PMC article.
-
R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission.Virology. 2007 Feb 5;358(1):55-68. doi: 10.1016/j.virol.2006.07.029. Epub 2006 Aug 24. Virology. 2007. PMID: 16934308
-
Human immunodeficiency virus-1 acquisition in genital mucosa: Langerhans cells as key-players.J Intern Med. 2009 Jan;265(1):18-28. doi: 10.1111/j.1365-2796.2008.02046.x. J Intern Med. 2009. PMID: 19093957 Review.
-
HIV-1 coreceptor usage, transmission, and disease progression.Curr HIV Res. 2003 Apr;1(2):217-27. doi: 10.2174/1570162033485357. Curr HIV Res. 2003. PMID: 15043204 Review.
Cited by
-
Antigen transfer and its effect on vaccine-induced immune amplification and tolerance.Theranostics. 2022 Aug 1;12(13):5888-5913. doi: 10.7150/thno.75904. eCollection 2022. Theranostics. 2022. PMID: 35966588 Free PMC article. Review.
-
Spermine and spermidine bind CXCR4 and inhibit CXCR4- but not CCR5-tropic HIV-1 infection.Sci Adv. 2023 Jul 7;9(27):eadf8251. doi: 10.1126/sciadv.adf8251. Epub 2023 Jul 5. Sci Adv. 2023. PMID: 37406129 Free PMC article.
-
Autophagy-enhancing drugs limit mucosal HIV-1 acquisition and suppress viral replication ex vivo.Sci Rep. 2021 Feb 26;11(1):4767. doi: 10.1038/s41598-021-84081-4. Sci Rep. 2021. PMID: 33637808 Free PMC article.
-
Caveolin-1 mediated uptake via langerin restricts HIV-1 infection in human Langerhans cells.Retrovirology. 2014 Dec 31;11:123. doi: 10.1186/s12977-014-0123-7. Retrovirology. 2014. PMID: 25551286 Free PMC article.
-
New HIV-1 circulating recombinant form 94: from phylogenetic detection of a large transmission cluster to prevention in the age of geosocial-networking apps in France, 2013 to 2017.Euro Surveill. 2019 Sep;24(39):1800658. doi: 10.2807/1560-7917.ES.2019.24.39.1800658. Euro Surveill. 2019. PMID: 31576801 Free PMC article.
References
-
- WHO | HIV/AIDS. [ http://www.who.int/gho/hiv/en/]
-
- Berger EA, Doms RW, Fenyö EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. A new classification for HIV-1. Nature. 1998;391:240. - PubMed
-
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. - PubMed
-
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. - PubMed
-
- Schuitemaker H, Koot M, Kootstra NA, Dercksen MW, de Goede RE, van Steenwijk RP, Lange JM, Schattenkerk JK, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

