Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease
- PMID: 24990275
- PMCID: PMC4310398
- DOI: 10.1161/JAHA.114.001072
Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease
Erratum in
- J Am Heart Assoc. 2014 Aug;3(4):e000456
Abstract
Background: NOX-2, the catalytic subunit of NADPH oxidase, has a key role in the formation of reactive oxidant species and is implicated in impairing flow-mediated dilation (FMD). Dark chocolate exerts artery dilatation via down-regulating NOX2-mediated oxidative stress. The aim of this study was to investigate whether dark chocolate improves walking autonomy in peripheral artery disease (PAD) patients via an oxidative stress-mediated mechanism.
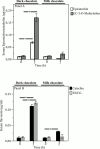
Methods and results: FMD, serum levels of isoprostanes, nitrite/nitrate (NOx) and sNOX2-dp, a marker of blood NOX2 activity, maximal walking distance (MWD) and maximal walking time (MWT) were studied in 20 PAD patients (14 males and 6 females, mean age: 69±9 years) randomly allocated to 40 g of dark chocolate (>85% cocoa) or 40 g of milk chocolate (≤35% cocoa) in a single blind, cross-over design. The above variables were assessed at baseline and 2 hours after chocolate ingestion. Dark chocolate intake significantly increased MWD (+11%; P<0.001), MWT (+15%; P<0.001), serum NOx (+57%; P<0.001) and decreased serum isoprostanes (-23%; P=0.01) and sNOX2-dp (-37%; P<0.001); no changes of the above variables were observed after milk chocolate intake. Serum epicatechin and its methylated metabolite significantly increased only after dark chocolate ingestion. Multiple linear regression analysis showed that Δ of MWD was independently associated with Δ of MWT (P<0.001) and Δ of NOx (P=0.018). In vitro study demonstrated that HUVEC incubated with a mixture of polyphenols significantly increased nitric oxide (P<0.001) and decreased E-selectin (P<0.001) and VCAM1 (P<0.001).
Conclusion: In PAD patients dark but not milk chocolate acutely improves walking autonomy with a mechanism possibly related to an oxidative stress-mediated mechanism involving NOX2 regulation.
Clinical trial registration url: http://www.clinicaltrials.gov. Unique identifier: NCT01947712.
Keywords: antioxidant; atherosclerosis; chocolate; oxidant stress; peripheral vascular disease.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures



Comment in
-
[Chocolate relieves peripheral artery disease?].MMW Fortschr Med. 2014 Oct 9;156(17):41. MMW Fortschr Med. 2014. PMID: 25417468 German. No abstract available.
References
-
- Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:2851-2906. - PubMed
-
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Transatlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation. 2006; 113:e463-e654. - PubMed
-
- Brevetti G, Corrado S, Martone VD, Di Donato A, Silvestro A, Vanni L. Microcirculation and tissue metabolism in peripheral arterial disease. Clin Hemorheol Microcirc. 1999; 21:245-254. - PubMed
-
- Coutinho T, Rooke TW, Kullo IJ. Arterial dysfunction and functional performance in patients with peripheral artery disease: a review. Vasc Med. 2011; 16:203-211. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

