Endothelin-converting enzyme 2 differentially regulates opioid receptor activity
- PMID: 24990314
- PMCID: PMC4292980
- DOI: 10.1111/bph.12833
Endothelin-converting enzyme 2 differentially regulates opioid receptor activity
Abstract
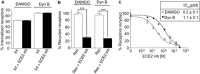
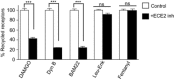
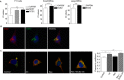
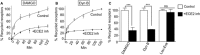
Background and purpose: Opioid receptor function is modulated by post-activation events such as receptor endocytosis, recycling and/or degradation. While it is generally understood that the peptide ligand gets co-endocytosed with the receptor, relatively few studies have investigated the role of the endocytosed peptide and peptide processing enzymes in regulating receptor function. In this study, we focused on endothelin-converting enzyme 2 (ECE2), a member of the neprilysin family of metallopeptidases that exhibits an acidic pH optimum, localizes to an intracellular compartment and selectively processes neuropeptides including opioid peptides in vitro, and examined its role in modulating μ receptor recycling and resensitization.
Experimental approach: The effect of ECE2 inhibition on hydrolysis of the endocytosed peptide was examined using thin-layer chromatography and on μ opioid receptor trafficking using either elisa or microscopy. The effect of ECE2 inhibition on receptor signalling was measured using a cAMP assay and, in vivo, on antinociception induced by intrathecally administered opioids by the tail-flick assay.
Key results: The highly selective ECE2 inhibitor, S136492, significantly impaired μ receptor recycling and signalling by only those ligands that are ECE2 substrates and this was seen both in heterologous cells and in cells endogenously co-expressing μ receptors with ECE2. We also found that ECE2 inhibition attenuated antinociception mediated only by opioid peptides that are ECE2 substrates.
Conclusions and implications: These results suggest that ECE2, by selectively processing endogenous opioid peptides in the endocytic compartment, plays a role in modulating opioid receptor activity.
Linked articles: This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2.
© 2014 The British Pharmacological Society.
Figures








Similar articles
-
Opioid receptor function is regulated by post-endocytic peptide processing.J Biol Chem. 2014 Jul 11;289(28):19613-26. doi: 10.1074/jbc.M113.537704. Epub 2014 May 20. J Biol Chem. 2014. PMID: 24847082 Free PMC article.
-
Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins.J Cell Biol. 2007 Dec 3;179(5):981-97. doi: 10.1083/jcb.200704053. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039931 Free PMC article.
-
Change in functional selectivity of morphine with the development of antinociceptive tolerance.Br J Pharmacol. 2015 Jan;172(2):549-61. doi: 10.1111/bph.12703. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24666417 Free PMC article.
-
Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities.Br J Pharmacol. 2015 Jan;172(2):375-87. doi: 10.1111/bph.12663. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24571499 Free PMC article. Review.
-
Different mechanisms of homologous and heterologous μ-opioid receptor phosphorylation.Br J Pharmacol. 2015 Jan;172(2):311-6. doi: 10.1111/bph.12627. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24517854 Free PMC article. Review.
Cited by
-
Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer's disease.Front Aging Neurosci. 2014 Sep 17;6:235. doi: 10.3389/fnagi.2014.00235. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25278875 Free PMC article. Review.
-
Distribution and trafficking of the μ-opioid receptor in enteric neurons of the guinea pig.Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1;311(2):G252-66. doi: 10.1152/ajpgi.00184.2016. Epub 2016 Jun 30. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27365337 Free PMC article.
-
Collybolide is a novel biased agonist of κ-opioid receptors with potent antipruritic activity.Proc Natl Acad Sci U S A. 2016 May 24;113(21):6041-6. doi: 10.1073/pnas.1521825113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162327 Free PMC article.
-
Compartment-specific opioid receptor signaling is selectively modulated by different dynorphin peptides.Elife. 2021 Apr 28;10:e60270. doi: 10.7554/eLife.60270. Elife. 2021. PMID: 33908346 Free PMC article.
-
Delta opioid receptors recycle to the membrane after sorting to the degradation path.Cell Mol Life Sci. 2018 Jun;75(12):2257-2271. doi: 10.1007/s00018-017-2732-5. Epub 2017 Dec 29. Cell Mol Life Sci. 2018. PMID: 29288293 Free PMC article.
References
-
- Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, Woods JH. Stimulation of guanosine-5'-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286:282–288. - PubMed
-
- Chavkin C, Henriksen SJ, Siggins GR, Bloom FE. Selective inactivation of opioid receptors in rat hippocampus demonstrates that dynorphin-A and -B may act on mu-receptors in the CA1 region. Brain Res. 1985;331:366–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

