A 3D in vitro cancer model as a platform for nanoparticle uptake and imaging investigations
- PMID: 24990320
- PMCID: PMC4282585
- DOI: 10.1002/smll.201400194
A 3D in vitro cancer model as a platform for nanoparticle uptake and imaging investigations
Abstract
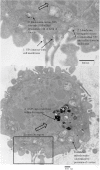
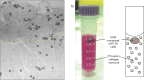
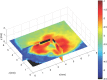
In order to maximize the potential of nanoparticles (NPs) in cancer imaging and therapy, their mechanisms of interaction with host tissue need to be fully understood. NP uptake is known to be dramatically influenced by the tumor microenvironment, and an imaging platform that could replicate in vivo cellular conditions would make big strides in NP uptake studies. Here, a novel NP uptake platform consisting of a tissue-engineered 3D in vitro cancer model (tumoroid), which mimics the microarchitecture of a solid cancer mass and stroma, is presented. As the tumoroid exhibits fundamental characteristics of solid cancer tissue and its cellular and biochemical parameters are controllable, it provides a real alternative to animal models. Furthermore, an X-ray fluorescence imaging system is developed to demonstrate 3D imaging of GNPs and to determine uptake efficiency within the tumoroid. This platform has implications for optimizing the targeted delivery of NPs to cells to benefit cancer diagnostics and therapy.
Keywords: 3D in vitro model; X-ray fluorescence; cancer model; gold nanoparticles; imaging.
© 2014 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

