Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming
- PMID: 24990442
- PMCID: PMC4198042
- DOI: 10.15252/embr.201438463
Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming
Abstract
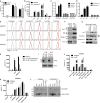
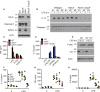
A current paradigm proposes that mitochondrial damage is a critical determinant of NLRP3 inflammasome activation. Here, we genetically assess whether mitochondrial signalling represents a unified mechanism to explain how NLRP3 is activated by divergent stimuli. Neither co-deletion of the essential executioners of mitochondrial apoptosis BAK and BAX, nor removal of the mitochondrial permeability transition pore component cyclophilin D, nor loss of the mitophagy regulator Parkin, nor deficiency in MAVS affects NLRP3 inflammasome function. In contrast, caspase-8, a caspase essential for death-receptor-mediated apoptosis, is required for efficient Toll-like-receptor-induced inflammasome priming and cytokine production. Collectively, these results demonstrate that mitochondrial apoptosis is not required for NLRP3 activation, and highlight an important non-apoptotic role for caspase-8 in regulating inflammasome activation and pro-inflammatory cytokine levels.
Keywords: NLRP3; apoptosis; caspase‐8; inflammasome; mitochondria.
© 2014 The Authors.
Figures


References
-
- Croker BA, O’Donnell JA, Gerlic M. Pyroptotic death storms and cytopenia. Curr Opin Immunol. 2014;26:128–137. - PubMed
-
- Khan N, Lawlor KE, Murphy JM, Vince JE. More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr Opin Immunol. 2014;26:76–89. - PubMed
-
- Lawlor KE, Vince JE. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim Biophys Acta. 2013;1840:1433–1440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

